Inhaltsverzeechnes
D'Evaluatioun vun enger Endoskopiefabréck erfuerdert e Kader, deen d'Konformitéit mat de Reglementer, d'Produktiounskontrollen, d'Ingenieursfäegkeeten an d'Liwwerantmanagement bewäert. Fir d'Beschaffung vu Spideeler a medizinesch Distributeuren garantéiert dës Due Diligence d'Patientensécherheet, d'Zouverlässegkeet vun den Apparater an optimal Gesamtbesëtzkäschten. Dëse Guide beschreift déi wichtegst Piliere fir d'Auditéiere vun de Qualitéitssystemer an der laangfristeger Rentabilitéit vun engem potenziellen Produktiounspartner, andeems en iwwer d'Spezifikatioune erausgeet a sech op fundamental Prozesser konzentréiert.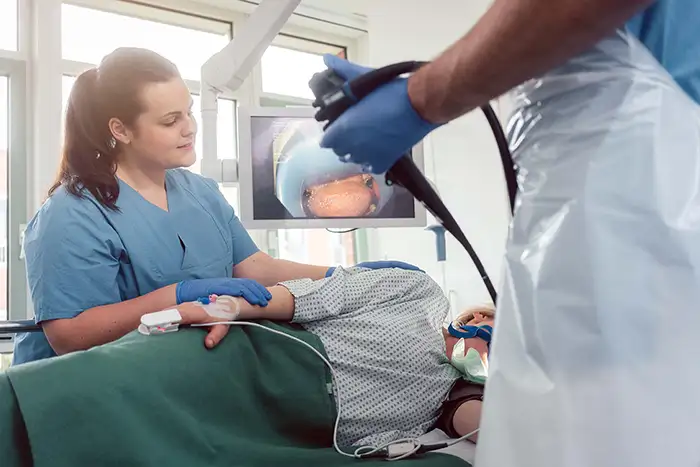
D'Bewäertung vun der Produktiounsexzellenz erfuerdert eng grëndlech Evaluatioun vun de fundamentale Qualitéitssystemer a Produktiounsstandarden.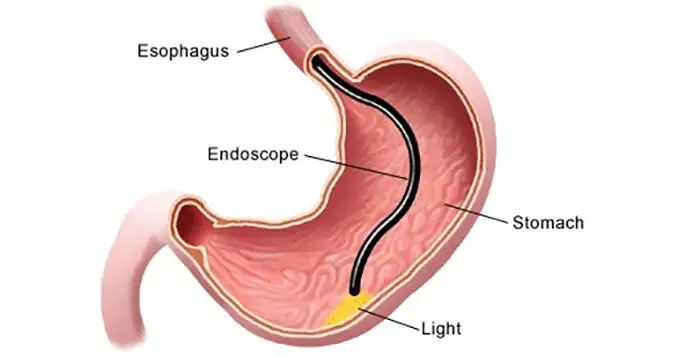
Gëlteg ISO 13485 Zertifizéierung fir d'Produktioun vu medizineschen Apparater
Erfollegräich FDA-Registréierung an Dokumentatioun vum Maartgenehmigung
EU MDR-Konformitéit a Virbereedung vun techneschen Dossieren
International elektresch Sécherheetsnormen, dorënner d'IEC 60601 Serie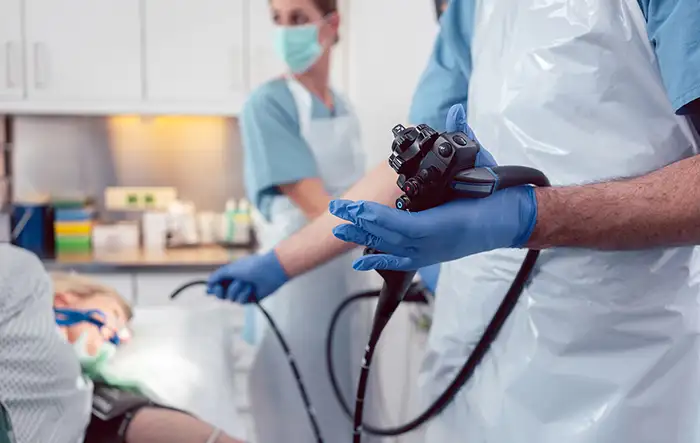
Zertifizéiert Klassifikatioun a Wartungsprotokoller fir propper Raim
Ëmweltiwwerwaachungssystemer fir Temperatur- a Fiichtegkeetskontroll
Moossname fir d'Verhënnerung vu partikelfërmegen Kontaminatioun
Sterilisatiounsvalidatioun an Integritéitstester vun der Verpackung
D'Produktiounsqualitéit geet iwwer d'Konformitéit eraus a ëmfaasst och technesch Expertise a Innovatiounskapazitéit.
Zesummesetzung an Expertise vun engem multidisziplinäre Ingenieursteam
Ëmsetzung an Dokumentatioun vum Designkontrollprozess
Methodologie vum Risikomanagement no ISO 14971
Prototyping-Fäegkeeten a Verifizéierungstestprotokoller
Ëmsetzung vun automatiséierten opteschen Inspektiounssystemer
Präzisiounsbearbechtung a Montagetechniken
Roboterhëllef bei komplexen Montageoperatiounen
Echtzäit Produktiounsiwwerwaachung an Datenerfassung
Eng ëmfaassend Qualitéitssécherung erfuerdert Exzellenz an der ganzer Liwwerkette an am Produktiounsökosystem.
Spezifikatioun a Verifizéierungsprozesser fir Rohmaterialien
Prozedure fir d'Auditéiere vu Fournisseuren a Leeschtungsiwwerwaachung
Komponentenverfolgungssystemer a Chargekontroll
Protokoller fir d'Inspektioun vun den Entréeën an Akzeptanzkriterien
Qualitéitskontrollpunkten am Prozess
Ëmsetzung vu statistesche Prozesskontrollen
Testen a Validatioun vun der Endproduktioun
Net-konform Materialbehandlungsprozeduren
Nohalteg Produktiounsqualitéit weist Engagement duerch kontinuéierlech Ënnerstëtzung a systematesch Verbesserung.
Disponibilitéit vum globale Netzwierk vun der technescher Ënnerstëtzung
Reparatur- a Wartungsserviceméiglechkeeten
Ressourcen fir klinesch Ausbildung a Bildung
Ersatzdeeler Lagerverwaltung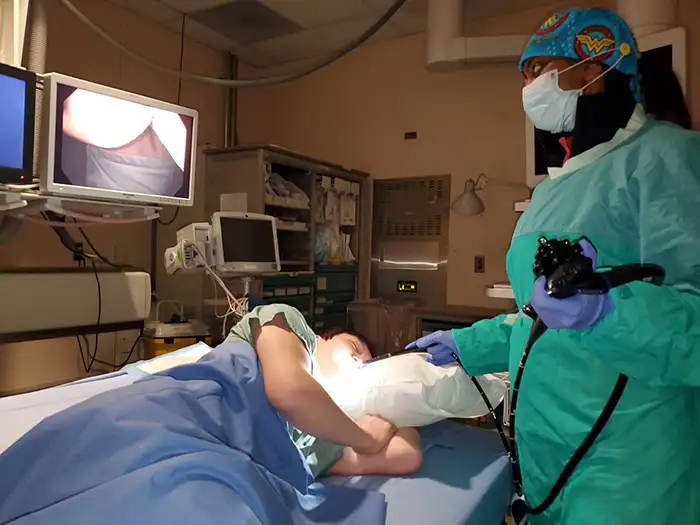
Ëmsetzung vum Post-Maart-Iwwerwaachungssystem
Sammlung an Analyse vu Clientfeedback
Tracking vun de Leeschtungsmetriken am Feld
Dokumentatioun vum Prozess vun der kontinuéierlecher Verbesserung
Eng ëmfaassend Evaluatioun vun enger Endoskopiefabréck erfuerdert eng Bewäertung iwwer verschidde Dimensiounen vun der Produktiounsexzellenz. Dësen strukturéierten Usaz erméiglecht informéiert Partnerschaftsentscheedungen op Basis vun nogewisenen Fäegkeeten a laangfristeger Qualitéitsleistung.
Copyright © 2025.Geekvalue All Rechter reservéiert.Techneschen Support: TiaoQingCMS