ކޮންޓެންޓްސް ތާވަލް
އެންޑޯސްކޯޕީ ފެކްޓްރީއެއް އިވެލުއޭޓް ކުރާނެ ގޮތް ރެގިއުލޭޓަރީ ކޮމްޕްލައިންސް، ޕްރޮޑަކްޝަން ކޮންޓްރޯލްސް، އިންޖިނިއަރިން ކެޕޭސިޓީ، އަދި ސަޕްލައި މެނޭޖްމަންޓް ވަޒަންކުރާ އޮނިގަނޑެއް ބޭނުންވެއެވެ. ހޮސްޕިޓަލް ޕްރޮކިއުމަންޓާއި މެޑިކަލް ޑިސްޓްރިބިއުޓަރުންނަށް މި ޑިއު ޑިލިޖެންސްގެ ސަބަބުން ބަލިމީހުންގެ ރައްކާތެރިކަމާއި، ޑިވައިސްގެ އިތުބާރު ކުރެވޭ ގޮތާއި، މިލްކުވެރިކަމުގެ ޖުމްލަ ޚަރަދު އެންމެ ރަނގަޅުކަން ކަށަވަރުވެގެންދެއެވެ. މި ގައިޑްގައި ސްޕެސިފިކޭޝަންތަކުން ކުރިއަށް ގޮސް ފައުންޑޭޝަނަލް ޕްރޮސެސްތަކަށް ގޮސް، ޕޮޓެންޝަލް މެނުފެކްޗަރިންގ ޕާޓްނަރެއްގެ ކޮލިޓީ ސިސްޓަމްތަކާއި ދިގުމުއްދަތުގެ ވިއޭބިލިޓީ އޮޑިޓްކުރުމަށް މުހިންމު ތަނބުތައް ބަޔާންކޮށްފައިވެއެވެ.
އުފެއްދުންތެރިކަމުގެ މޮޅުކަން ވަޒަންކުރުމަށްޓަކައި އަސާސީ ކޮލިޓީ ސިސްޓަމްތަކާއި އުފެއްދުމުގެ މިންގަނޑުތައް ފުރިހަމައަށް އިވެލުއޭޓް ކުރަން ޖެހެއެވެ.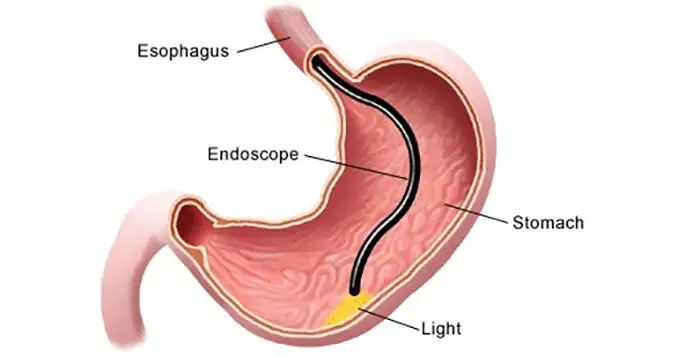
މެޑިކަލް ޑިވައިސް އުފެއްދުމުގެ ނިޒާމުތަކަށް ސައްހަ އައި.އެސް.އޯ 13485 ސެޓްފިކެޓް ލިބިފައިވުން
ކާމިޔާބު އެފްޑީއޭ ރަޖިސްޓްރޭޝަން އަދި މާކެޓް ކްލިއަރެންސް ޑޮކިއުމަންޓޭޝަން ހޯދުން
އީޔޫ އެމްޑީއާރު ކޮމްޕްލައިންސް އާއި ޓެކްނިކަލް ފައިލް ތައްޔާރުކުރުން
އައިއީސީ 60601 ސީރީޒް ހިމެނޭ ގޮތަށް ބައިނަލްއަގްވާމީ އިލެކްޓްރިކަލް ސޭފްޓީ ސްޓޭންޑަޑްސް
ސެޓްފިކެޓް ލިބިފައިވާ ކްލީންރޫމް ކްލަސިފިކޭޝަން އަދި މެއިންޓެނަންސް ޕްރޮޓޮކޯލްތައް
ފިނިހޫނުމިނާއި ހޫނުމިން ކޮންޓްރޯލް ކުރުމަށް ތިމާވެށި މޮނިޓަރ ކުރުމުގެ ނިޒާމުތައް ގާއިމްކުރުން
ޕާޓިކިއުލެޓް ތަޣައްޔަރުވުން ހުއްޓުވުމަށް އަޅަންޖެހޭ ފިޔަވަޅުތައް
ސްޓިރިލައިޒޭޝަން ވެލިޑޭޝަން އަދި ޕެކޭޖިންގ އިންޓެގްރިޓީ ޓެސްޓްކުރުން
އުފެއްދުންތެރިކަމުގެ ފެންވަރު ކޮމްޕްލައިންސް އަށް ވުރެ ވެސް ފުޅާވެ، ޓެކްނިކަލް ތަޖުރިބާއާއި އިނޮވޭޝަން ކެޕޭސިޓީ ހިމެނެ އެވެ.
މަލްޓިޑިސިޕްލިނަރީ އިންޖިނިއަރިންގ ޓީމް ކޮމްޕޮޒިޝަން އަދި ތަޖުރިބާ ލިބިފައިވުން
ޑިޒައިން ކޮންޓްރޯލް ޕްރޮސެސް އިމްޕްލިމެންޓޭޝަން އަދި ޑޮކިއުމަންޓޭޝަން
އައިއެސްއޯ 14971 އާ އެއްގޮތަށް ރިސްކް މެނޭޖްމަންޓް މެތޮޑޮލޮޖީ
ޕްރޮޓޯޓައިޕިންގ ކެޕޭސިޓީސް އަދި ވެރިފިކޭޝަން ޓެސްޓިންގ ޕްރޮޓޮކޯލްސް
އޮޓޮމެޓިކް އޮޕްޓިކަލް އިންސްޕެކްޝަން ސިސްޓަމްތައް ތަންފީޒުކުރުން
ޕްރެސިޝަން މެޝިނިންގ އަދި އެސެމްބްލީ ޓެކްނިކްސް
ކޮމްޕްލެކްސް އެސެމްބްލީ އޮޕަރޭޝަންތަކުގައި ރޮބޮޓިކް އެހީތެރިކަން ފޯރުކޮށްދިނުން
ރިއަލް ޓައިމް ޕްރޮޑަކްޝަން މޮނިޓަރިންގ އާއި ޑޭޓާ އެއްކުރުން
ފުރިހަމަ ކޮލިޓީ އެޝުއަރެންސް އަށް މުޅި ސަޕްލައި ޗޭން އާއި މެނުފެކްޗަރިން އިކޯސިސްޓަމްގައި ވެސް މޮޅުކަން ބޭނުންވެ އެވެ.
ރޯ މެޓީރިއަލް ސްޕެސިފިކޭޝަން އަދި ވެރިފިކޭޝަން ޕްރޮސެސްތައް
ސަޕްލައިއަރ އޮޑިޓް ޕްރޮސީޖަރސް އަދި ޕާފޯމަންސް މޮނިޓަރިންގ
ކޮމްޕޮނެންޓް ޓްރޭސެބިލިޓީ ސިސްޓަމްތަކާއި ލޮޓް ކޮންޓްރޯލް ކުރުން
އިންކަމް އިންސްޕެކްޝަން ޕްރޮޓޮކޯލްތަކާއި އެކްސެޕްޓެންސް ކްރައިޓީރިއާ
އިން-ޕްރޮސެސް ކޮލިޓީ ކޮންޓްރޯލް ޗެކްޕޮއިންޓްތައް
ތަފާސްހިސާބު ޕްރޮސެސް ކޮންޓްރޯލް ތަންފީޒުކުރުން
ފައިނަލް ޕްރޮޑަކްޓް ޓެސްޓިންގ އަދި ޕާފޯމަންސް ވެލިޑޭޝަން
ނޮން ކޮންފޯމިންގ މެޓީރިއަލް ހެންޑްލިންގ ޕްރޮސީޖަރސް
ދެމެހެއްޓެނިވި ގޮތެއްގައި އުފެއްދުންތެރިކަމުގެ ފެންވަރަކީ ކުރިއަށްދާ ސަޕޯޓާއި ނިޒާމީ ގޮތުން ރަނގަޅުކުރުމުގެ ތެރެއިން ކޮމިޓްމަންޓް ދައްކުވައިދޭ ކަމެކެވެ.
ގްލޯބަލް ޓެކްނިކަލް ސަޕޯޓް ނެޓްވޯކް ލިބޭނެ ގޮތް ހެދުން
މަރާމާތުކުރުމާއި ބެލެހެއްޓުމުގެ ޚިދުމަތުގެ ޤާބިލުކަން
ކްލިނިކަލް ޓްރެއިނިންގ އަދި ތަޢުލީމީ ވަސީލަތްތައް
ސްޕެއާ ޕާޓްސް އިންވެންޓްރީ ބެލެހެއްޓުން
ޕޯސްޓް މާކެޓް ސަރވޭލަންސް ސިސްޓަމް ތަންފީޒުކުރުން
ކަސްޓަމަރުންގެ ފީޑްބެކް އެއްކުރުމާއި ތަޙުލީލުކުރުން
ފީލްޑް ޕާފޯމަންސް މެޓްރިކްސް ޓްރެކިންގ
މެދުނުކެނޑި ރަނގަޅު ކުރުމުގެ ޕްރޮސެސް ޑޮކިއުމަންޓޭޝަން
އެންޑޯސްކޯޕީ ފެކްޓްރީއެއްގެ ފުރިހަމަ އިވެލުއޭޝަން ހެދުމަށްޓަކައި އުފެއްދުންތެރިކަމުގެ މޮޅުކަމުގެ އެތައް ޑިމޭންޝަންތަކެއް ހުރަސްކޮށް ވަޒަންކުރަން ޖެހެއެވެ. މި ސްޓްރަކްޗަރޑް އެޕްރޯޗަކީ ދައްކާފައިވާ ޤާބިލުކަމާއި ދެމެހެއްޓެނިވި ފެންވަރު ރަނގަޅު ޕާފޯމަންސްގެ މައްޗަށް ބިނާކޮށް މަޢުލޫމާތު ލިބިފައިވާ ޕާޓްނަރޝިޕް ނިންމުންތައް ނިންމުމަށް މަގުފަހިކޮށްދޭ އުސޫލެކެވެ.
ކޮޕީރައިޓް © 2025.ގީކްވެލިއު ހުރިހާ ހައްޤުތަކެއް ލިބިގެންވެއެވެ.ޓެކްނިކަލް ސަޕޯޓް: TiaoQingCMS