Table of Contents
A cystoscope is a specialized endoscopic instrument used to directly visualize the urethra and bladder for diagnosis and treatment. Inserted through the urethral opening, a cystoscope carries illumination and either fiber-optic bundles or a digital sensor to relay high-resolution images. By providing real-time views of mucosa, lesions, and devices inside the lower urinary tract, a cystoscope enables targeted biopsies, stone retrieval, tumor resection support, and stent manipulation—often in the same session—reducing uncertainty, shortening clinical pathways, and improving outcomes.
When patients present with hematuria, recurrent infections, lower urinary tract symptoms, unexplained pelvic pain, or a history of bladder cancer, speed and accuracy are critical. Imaging such as ultrasound and CT can suggest abnormalities, but they cannot replace the direct view a cystoscope provides. Cystoscopy clarifies whether a shadow is a lesion or a fold, whether a stone is embedded or mobile, and whether a stricture is short, ring-like, or long segment. This fidelity drives correct staging, appropriate therapy, and efficient follow-up.
Direct visualization improves diagnostic certainty and guides immediate intervention.
Combined diagnosis and treatment in a single encounter reduces anesthesia exposures.
Real-time documentation supports team communication, teaching, and quality improvement.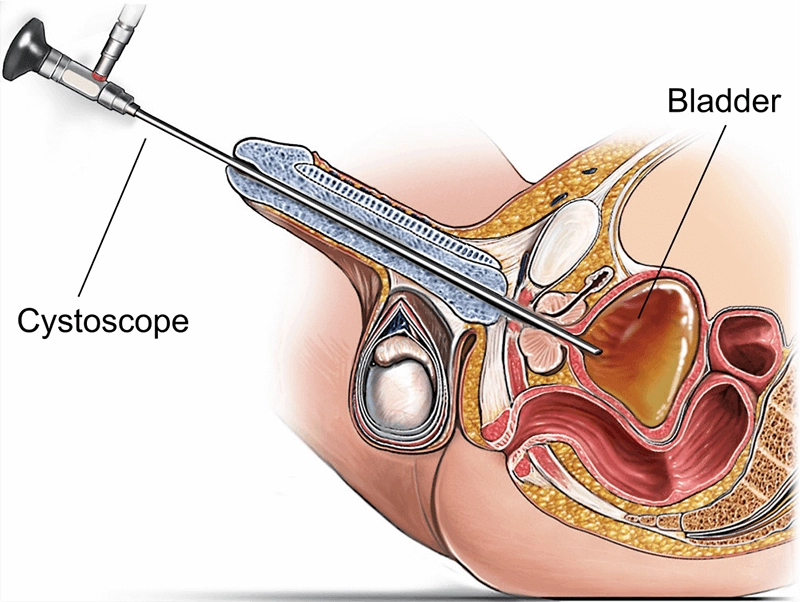
Late-19th-century pioneers proved that light and lenses could make the urinary tract visible, though early devices were rigid, bulky, and dim. Mid-20th-century fiber optics improved brightness and flexibility, enabling office-based diagnostic cystoscopy. The adoption of chip-on-tip digital sensors brought high-definition images, better low-light performance, and reliable recording. More recently, single-use cystoscopes have expanded options for infection control and fast turnaround in high-throughput settings.
Fiber-optic era: coherent bundles carried images to an eyepiece but were prone to “black dots” from fiber breakage.
Digital video era: distal CMOS sensors provided HD, color fidelity, and easy recording for training and QA.
Disposable pathways: eliminated reprocessing steps at the expense of per-case consumable cost and waste.
Lower urinary tract anatomy dictates scope diameter, flexibility, and maneuvering strategy. In males, curvature and sphincter tone make gentle, well-lubricated advancement essential; in females, the urethra is shorter and straighter but requires meticulous asepsis. In the bladder, a systematic survey covers trigone, ureteric orifices, interureteric ridge, dome, posterior, lateral, and anterior walls.
Male urethra: meatus → fossa navicularis → penile → bulbar → membranous → prostatic urethra → bladder neck.
Female urethra: shorter course with different angulation and infection-prevention priorities.
Bladder landmarks: trigone, ureteric orifices, interureteric ridge, and dome require adequate distension and angulation.
Insertion tube and sheath: biocompatible, kink-resistant, sized for comfort and access through strictures.
Optics and imaging: fiber bundles or distal CMOS; anti-fog, hydrophilic, or scratch-resistant windows.
Illumination: LED sources with adjustable intensity for pale or hemorrhagic fields.
Deflection and steering: control wheels for up/down (and sometimes lateral) deflection in flexible scopes.
Working channels and irrigation: instrument passage and steady distension; dual channels improve stability.
Handle and UI: ergonomic grips, capture/freeze buttons, and cable management for low-fatigue control.
Connectivity: monitors/processors with image storage, DICOM export, and secure network integration.
Rigid cystoscope: excellent optics and robust channels; often used for operative workflows (e.g., TURBT support, stone work).
Flexible cystoscope: greater comfort and reach; ideal for office diagnostics and surveillance.
Video (chip-on-tip) cystoscope: HD imaging and recording for team situational awareness and teaching.
Single-use cystoscope: infection-control advantage and predictable availability; higher per-case consumable cost.
Pediatric variants: reduced diameters, gentler curves, and compatible micro-instruments.
Visible or microscopic hematuria workup to localize bleeding and rule out malignancy.
Bladder cancer surveillance to detect recurrence and guide intravesical therapy.
Recurrent urinary tract infections to identify stones, diverticula, or foreign bodies.
Lower urinary tract symptoms to exclude mechanical obstruction or intravesical lesions.
Urethral stricture evaluation to define site, length, and caliber for intervention planning.
Foreign body retrieval, stent placement, and removal.
Assessment after pelvic surgery or radiation for fistulae, necrosis, or radiation cystitis.
Explain goals (diagnostic vs potential treatment), steps, sensations, and likely post-procedure symptoms.
Review history, allergies, medications, and culture results; manage anticoagulation and antibiotics per policy.
Check equipment readiness: scope integrity, instrument sets, irrigation, and recording systems.
Position (lithotomy or dorsal recumbent), sterile prep, and gel anesthetic as indicated.
Advance under direct vision; never force past resistance.
Maintain uniform distension with isotonic irrigation; perform a systematic bladder survey.
Intervene as planned (biopsy, hemostasis, stone retrieval, stent tasks) and document with images.
Encourage hydration; provide analgesia guidance and red-flag symptoms (fever, retention, heavy clots).
Schedule follow-up for pathology, surveillance intervals, and symptom reassessment.
Begin with panoramic sweeps; adjust light/gain; rotate to maintain spatial orientation.
Characterize lesions by size, color, vascularity, contour, borders, and proximity to orifices.
Use appropriately sized biopsy forceps; label specimens by precise location.
Consider digital contrast or fluorescence modes (where available) to improve detection of subtle flat lesions.
TURBT support: map lesions, biopsy edges, identify satellites; document with clock-face orientation.
Stone management: basket small calculi; fragment larger stones (ultrasonic, pneumatic, laser) and retrieve fragments.
Stricture management: define anatomy; perform dilation or incision when appropriate; plan urethroplasty for longer segments.
Hemostasis: pinpoint bleeding control with conservative energy settings and clear visualization.
Stent work: precise placement and removal with stable view of trigone and orifices.
UTI: reduce with proper selection, sterile technique, and reprocessing discipline; evaluate persistent fever or flank pain.
Hematuria: usually self-limited; give hydration and return precautions.
Perforation: rare; avoid blind force, especially in strictures; manage from catheter drainage to repair based on severity.
Pain/trauma: minimize by lubrication, correct size selection, and gentle handling.
Fluid overload: monitor inflow/outflow in long resections; use isotonic irrigation when compatible with energy modality.
Point-of-use care: pre-clean to prevent biofilm; leak test before immersion.
Manual cleaning: enzymatic detergents and channel brushing per IFU.
High-level disinfection or sterilization: validated chemistries or low-temperature systems; complete drying and protected storage.
Automation: AERs standardize parameters; training and audits sustain compliance.
Single-use option: useful where reprocessing capacity is limited or outbreak control is paramount.
Resolution/dynamic range: preserve detail in bright reflections and shaded recesses.
Color truth/white balance: accurate color helps distinguish inflammation from neoplasia.
Image stability: ergonomic design, smooth deflection, anti-fog coatings, and warmed irrigation.
Documentation: standard views of all regions and representative lesion images/clips.
Balanced grips, rotatable connectors, and micro-breaks reduce clinician fatigue.
Step-by-step narration and privacy assurances improve patient comfort and trust.
Analgesia ranges from topical gels and NSAIDs to minimal sedation for selected cases.
Office diagnostics volume, operative complexity, pediatric share, and cancer surveillance program.
Sensor generation, resolution, color stability, channel sizes, deflection range, outer diameters, illumination, and durability.
Capital cost vs lifespan, repair cycles, loaners, reprocessing costs, disposables vs reusables, service contracts, and updates.
Image capture/EHR connectivity, storage logistics, inventory, and staff training/competency validation.
Scheduled inspections for sheath wear, lens scratches, steering play, and connector integrity.
Leak testing to prevent fluid ingress and electronic damage.
Event logs tying each use to patient/operator; trend repairs to target retraining.
Processor firmware updates and monitor color calibration for consistent fidelity.
Office-based cystoscopy expands capacity beyond the OR and shortens wait times.
Reliable cancer surveillance reduces emergency presentations and aligns care with guidelines.
Robust reprocessing or selective single-use deployment reduces outbreak risk and service disruptions.
Pediatrics: smaller scopes, minimal trauma, family-centered communication, tailored sedation.
Neurogenic bladder: anticipate chronic inflammation and catheter-related changes; biopsy judiciously.
Anticoagulated patients: balance bleeding and thrombotic risks; coordinate periprocedural plans.
Radiation cystitis: friable mucosa; conservative energy use and planned intravesical therapies.
Simulation, benchtop practice, and supervised cases build psychomotor skills.
Milestones: handling, systematic survey, lesion characterization, basic interventions.
Team training for nurses and reprocessing staff; cross-coverage maintains service continuity.
Audit with photo documentation, UTI rates, complications, and patient-reported outcomes.
AI-assisted detection: algorithms to flag subtle lesions and standardize reporting.
Spectral/fluorescence modes: digital contrast to improve sensitivity for flat lesions.
Smaller, smarter, greener: thinner scopes, efficient processors, and lifecycle-aware fleets.
Tele-support: secure live-view sharing for second opinions and remote education.
XBX positions its cystoscope portfolio around clarity, consistency, and continuity to align with real clinical workflows rather than one-off marketing features.
Clarity: emphasis on stable color, wide dynamic range, and anti-fog optics helps distinguish inflammation from suspicious flat lesions and map tumor borders confidently.
Consistency: ergonomic commonality across sizes/models reduces relearning; channel compatibility keeps instrument sets uniform; capture controls standardize documentation.
Continuity: installation training, refreshers for staff turnover, and service pathways prioritize uptime; mixed reusable/single-use strategies address infection control and scheduling needs.
By focusing on contribution rather than slogans, XBX supports urology teams in sustaining safe, reliable, and patient-centered cystoscopy programs over years of use.
The cystoscope remains a cornerstone of urology because it unites diagnostic certainty, therapeutic precision, and patient-centered efficiency in a single instrument. From rigid optics to flexible HD video and selective single-use options, its evolution has consistently expanded what clinicians can see and do without incisions. With disciplined reprocessing, thoughtful procurement, robust training, and contribution-oriented manufacturers such as XBX, cystoscopy will continue to anchor safe, timely, and effective care for bladder and urethral conditions in the decades ahead.
Cystoscopes are used for bladder cancer surveillance, hematuria investigation, stricture evaluation, stone management, and recurrent urinary tract infections.
Rigid cystoscopes provide excellent optics and robust channels, ideal for operative procedures, while flexible cystoscopes offer more comfort for patients and are often used in office diagnostics.
Video cystoscopes use chip-on-tip digital sensors to provide high-definition imaging, real-time documentation, and shared views for teaching and quality assurance.
Hospitals should follow strict reprocessing protocols, consider single-use cystoscopes when needed, and ensure leak testing, high-level disinfection, and proper storage to prevent contamination.
Key factors include image resolution, channel size, outer diameter for patient comfort, durability, cost of reprocessing, service support, and compatibility with hospital workflows.
Comfort is improved through topical anesthetic gels, lubrication, gentle insertion techniques, appropriate scope sizing, and clear communication with the patient.
Biopsy forceps, stone baskets, laser fibers, cautery electrodes, and stent graspers are among the instruments that can be passed through cystoscope working channels.
It enables early detection, mapping of tumor sites, targeted biopsies, and ongoing surveillance for recurrence, making it a gold standard in bladder cancer care.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS