Table of Contents
Reliable cystoscope sourcing supports medical efficiency and procurement accuracy. Choosing the right cystoscope factory ensures consistent quality, regulatory alignment, and supply chain trust.
Hospitals and healthcare procurement departments often face challenges when selecting a cystoscope factory. From technical standards to long-term cooperation models, a reliable manufacturer must align with not only product expectations but also with hospital protocols and global regulatory requirements. This guide explores key considerations for selecting a qualified cystoscope supplier or manufacturer and helps streamline the hospital procurement process effectively.
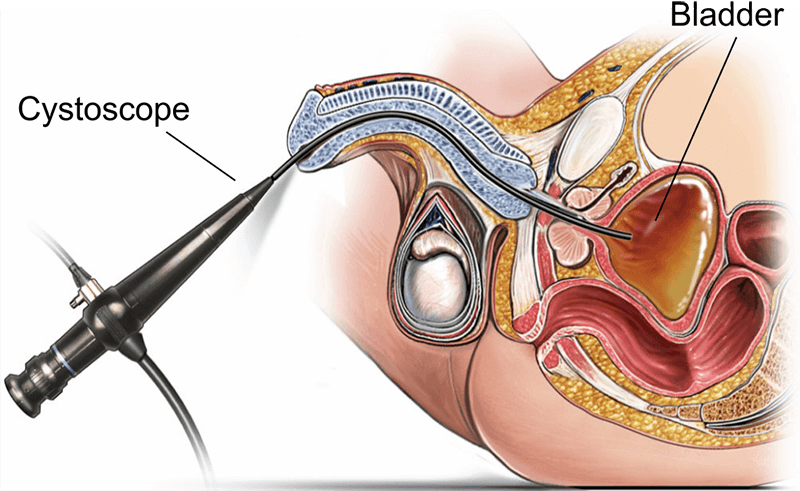
A trustworthy cystoscope factory is identified by its adherence to quality standards, certifications, and production transparency. Factories producing medical endoscopic devices must operate under strict medical device regulations. It's essential that manufacturing is carried out in controlled environments, with traceability across each unit, ensuring compatibility with hospital sterilization processes and patient safety protocols.
Beyond production quality, a factory’s history in medical device engineering plays a vital role. Long-term hospital procurement often favors factories that provide full technical documentation, support batch traceability, and offer stable logistics capabilities for international delivery. A capable cystoscope factory ensures flexibility for custom hospital needs, whether in specifications, connectors, or imaging system compatibility.
Cystoscope
Cystoscope manufacturers working in global markets must meet a range of hospital and regulatory compliance frameworks. This includes ISO standards, CE markings for European markets, and FDA registration for U.S.-based hospitals. However, compliance alone isn’t enough. Manufacturers must also maintain internal protocols that support cleanroom production, regular device validation, and ongoing quality audits.
Many hospitals evaluate manufacturers through structured technical documentation and sample evaluation. If a manufacturer can support test orders with clear sterilization compatibility, maintenance instructions, and warranty coverage documentation, they are often seen as prepared for hospital-level engagement. That said, manufacturers are rarely evaluated on product alone. Their ability to provide responsive post-purchase support often defines long-term value.

A cystoscope supplier plays a pivotal role as a logistics and communication bridge between the factory and the hospital. For many hospitals, especially those outside the manufacturer's region, working directly with a cystoscope supplier who understands local regulations, shipping logistics, and usage protocols ensures smoother procurement.
Effective suppliers provide procurement teams with accurate availability forecasts, detailed packing lists, sterilization guidelines, and importation documentation. Hospitals often request suppliers to coordinate calibration certificates, pre-shipment testing, and after-sales technical guidance. These services help procurement departments reduce uncertainty and streamline integration with existing endoscopic systems.
Moreover, a supplier’s ability to respond to technical inquiries and replacement requests affects hospital workflows. For recurring bulk orders, a responsive supplier becomes indispensable. Thus, reliability in communication and documentation holds as much weight as device quality itself.
Modern hospitals often seek custom solutions tailored to patient demographics, procedural needs, or internal systems. A forward-thinking cystoscope factory is prepared to support such requests without disrupting production timelines.
Whether it’s adjusting insertion tube lengths, integrating LED light sources, or modifying handles for ergonomic needs, factories offering modular production are preferred by procurement teams. Customizations also include labeling, packaging formats, and sterilization compatibility per region.
This capacity for customization allows hospitals to align devices with their surgical protocols and storage systems. It also supports training environments where standardized tools help medical teams practice with precision.
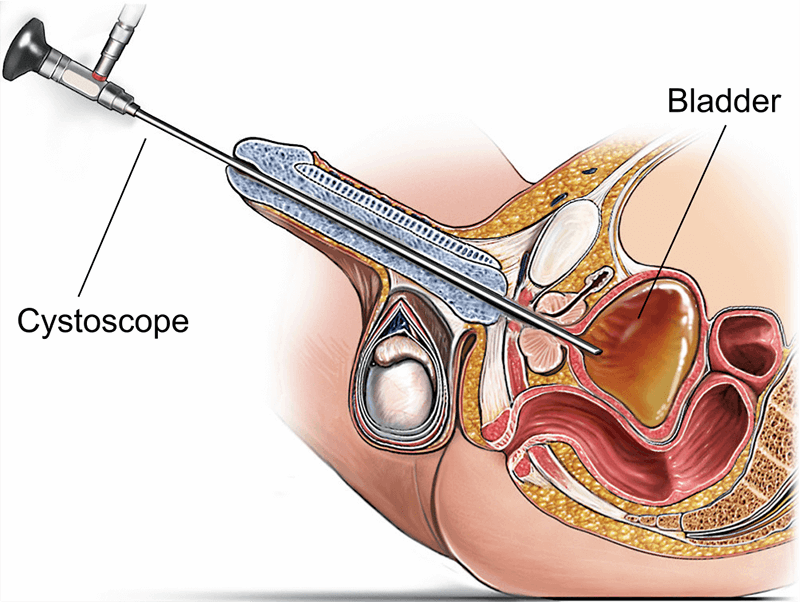
Traceability is crucial for both quality assurance and legal compliance. Cystoscope manufacturers must maintain unit-specific production logs, from material sourcing to final sterilization. Hospitals often require serialized labeling, barcoding, and digital records to align with their internal device tracking systems.
A reliable manufacturer integrates traceability not just as a quality step, but as a routine practice. With cloud-based tracking, many factories can now offer hospitals real-time visibility on order status and production stages. This minimizes delays and builds transparency into long-term partnerships.
Global healthcare systems vary in regulation, language, and customs handling. A cystoscope supplier suited for international markets is one that ensures multilingual documentation, global shipping experience, and certification familiarity.
Beyond that, international suppliers often handle specific hospital requirements, such as dual-voltage compatibility for imaging equipment or regional sterilization standards. Timely delivery is critical, especially when hospitals coordinate surgeries or new department launches based on incoming equipment.
Good suppliers also anticipate hospital questions before they arise. This might include providing instructional videos, usage manuals adapted to regional languages, or offering tele-support for installation and training.
Cystoscope pricing depends on several factors including design complexity, imaging quality, reusability, and supplier structure. Basic reusable cystoscopes might be priced more affordably, but long-term use requires sterilization investment and maintenance.
High-end systems with integrated cameras, advanced illumination, or wireless connectivity cost more and are typically purchased by tertiary care hospitals. Disposable cystoscopes are increasingly common in high-throughput departments aiming to reduce infection risks, although they come at a premium per use.
Additionally, procurement through a cystoscope supplier may include logistics, documentation, and tax handling fees. Hospitals often weigh upfront costs against service quality and long-term supplier reliability.
A cystoscope refers to the physical medical device — an endoscopic tool inserted through the urethra to visualize the bladder. It includes optical, lighting, and insertion components. A cystoscopy, on the other hand, is the clinical procedure in which a cystoscope is used.
Understanding the distinction is important for procurement teams. Hospitals purchase cystoscopes, but those purchases are tied to supporting cystoscopy procedures, which vary based on diagnostic or interventional needs. Thus, device design must match the procedural expectations of the medical team, including compatibility with irrigation systems, biopsy tools, or laser fibers.
Cystoscope
Hospital procurement is rarely about single transactions. Instead, it’s an ongoing relationship shaped by delivery reliability, technical improvements, and responsive support. Cystoscope manufacturers that continuously invest in product refinement, production automation, and post-market feedback channels are often favored by hospital systems seeking consistent quality over time.
Long-term collaboration also streamlines new product launches, allowing hospitals to adopt upgrades or innovations without re-validating the entire supply chain. Especially in regions with rapid technological advancement or regulatory updates, such partnerships ensure operational continuity.
When evaluating a cystoscope factory, hospital procurement officers must balance production capability, regulatory compliance, service quality, and adaptability. Similarly, manufacturers and suppliers need to support global healthcare expectations with structured documentation and technical alignment.
For hospitals seeking long-term, stable sourcing in the field of endoscopy and imaging devices, partnering with experienced industry names adds consistency to patient care and procedural efficiency.
XBX, as a dedicated brand in the medical endoscope field, supports hospitals and distributors globally with professional manufacturing and supply solutions designed for clinical application.
Hospitals should verify a cystoscope factory’s quality system beyond certificates—checking real implementation, CAPA discipline, supplier control, risk management, and traceability—so they partner with reliable cystoscope manufacturers and a dependable cystoscope supplier.
A trustworthy cystoscope factory shows proof, not just paperwork. Certificates matter, but procurement teams must see the systems that sustain quality day to day. Mature cystoscope manufacturers keep current, controlled documents and verifiable records that demonstrate how the organization turns procedures into consistent outcomes.
ECR/ECO logs that track design changes with cross-functional approvals.
Validated processes (IQ/OQ/PQ) for optical alignment, bending section assembly, and leak testing.
Routine in-process checkpoints tied to acceptance criteria and reaction plans.
Shop-floor access to the latest SOPs; obsolete versions archived and inaccessible.
When these artifacts are complete, date-stamped, and traceable to lots and serials, hospitals can trust the cystoscope supplier’s operational maturity rather than only a wall of certificates.
An effective CAPA program reveals culture. If leakage complaints cluster, the cystoscope factory should trace root causes—adhesive cure windows, O-ring variability, operator technique—then implement corrective and preventive actions, verify effectiveness, and close them on time. Use of 5-Why and fishbone methods with clear ownership shows the cystoscope supplier treats issues as opportunities to improve, not to hide.
Defined CAPA triggers and risk-based prioritization.
Root-cause evidence, not assumption.
Effectiveness checks with measurable criteria and due dates.
Management escalation for overdue actions.
Hospitals should review the complaint database and post-market surveillance plan. Robust cystoscope manufacturers trend minor signals, watch external vigilance notices, and run recall simulations to test readiness. If a recall occurred, the response timeline, documentation quality, and regulator communications indicate how the cystoscope supplier performs under pressure.
Complaint–batch–serial linkage with investigation notes.
Trend charts and thresholds that trigger CAPA.
Documented mock recalls with time-to-trace metrics.
Document control must follow ALCOA principles. Operators at a cystoscope factory should only see the latest SOPs. Batch records—electronic or paper—must be contemporaneous, legible, and attributable, with audit trails and compliant electronic signatures. This prevents after-the-fact entries and supports confidence in results reported by the cystoscope supplier.
Because sensors, optics, precision tubing, and biocompatible adhesives come from a global network, cystoscope manufacturers need strong supplier qualification and incoming quality control. Critical parts may require 100% inspection; others should use AQL-based sampling. Dual sourcing and supplier scorecards (rejection rate, on-time delivery, CAPA responsiveness) show whether the cystoscope factory can withstand shocks without compromising reliability.
Structured supplier onboarding and periodic audits.
Material certificates and traceable inspection results.
Clear nonconformance handling and supplier CAPA expectations.
ISO 14971 risk files should be living documents. Hazards such as cross-infection, leakage, or optical misalignment must map to risk controls that are verified and validated. When complaints arrive, dependable cystoscope manufacturers feed the information back into the risk file and reassess residual risk. This closed loop proves the cystoscope supplier manages real-world feedback—not just passes an audit once.
People make quality real. A cystoscope factory should maintain training matrices, certify operators for critical tasks (optical alignment, adhesive application, leak testing), and schedule re-certification. During audits, ask operators to explain key steps; confident, consistent answers often distinguish top-tier cystoscope manufacturers from those with only paper records.
Every device should be traceable from raw material to final test. A reliable cystoscope supplier assigns unique serials or UDI codes that your hospital can scan. Randomly select a finished scope and request its full genealogy—equipment IDs, process parameters, inspection results, and sign-offs. A cystoscope factory that retrieves this within minutes usually runs electronic batch records with secure audit trails, a strong predictor of recall readiness.
Lot-to-component linkage back to key suppliers.
Test data stored with timestamps and operator IDs.
UDI labeling aligned with regional regulations.
Ask to see the internal audit program: calendar, auditor qualifications, findings, and closures. Management review minutes should mention quality objectives, complaint trends, CAPA status, and resource allocation. When executives of the cystoscope factory attend these reviews and release budget or headcount to fix issues, you learn that quality is strategic—hallmark behavior among responsible cystoscope manufacturers.
Optical benches, leak testers, torque gauges, and environmental chambers must follow calibrated schedules traceable to national standards. If an instrument goes out of tolerance, the cystoscope supplier should quarantine potentially affected product, perform impact analysis, and document actions. This metrology discipline prevents silent drift in product performance.
Cystoscopes are sensitive to dust, humidity, and temperature. A credible cystoscope factory maintains controlled areas (often ISO Class 7 for optics), records particle counts, and controls environmental parameters that affect adhesive cure and polymer stability. Material flow segregates clean and dirty zones, and gowning procedures are enforced—habits common among leading cystoscope manufacturers.
Beyond compliance, look for signals of a learning organization: SPC charts on key parameters, first-pass yield dashboards, Kaizen events that remove waste, and Six Sigma projects targeting chronic defects. When a cystoscope supplier shows year-over-year reductions in rework and turnaround time, you gain confidence that today’s good results will be even better tomorrow.
If the cystoscope factory uses an electronic QMS, confirm controls for access, backups, disaster recovery, and audit trails. With rising cyber threats, protecting quality data is part of product integrity. Mature cystoscope manufacturers can describe how they test restorations and how quickly they can recover after a cyber incident.
Under EU MDR and FDA QSR, requirements evolve. Ask how the cystoscope supplier maintains PMCF/PMR activities, updates technical documentation, and prepares for inspections. Transparency about inspection history—plus timely, documented responses—signals that the cystoscope factory is confident in its system and honest with partners.
Observe a mock recall or a mock audit if possible. The best cystoscope manufacturers can identify affected lots within hours and show draft notifications and regulatory submissions. Watching the cystoscope supplier practice under time pressure is one of the fastest ways to assess real-world readiness.
An in-depth review helps hospitals separate marketing claims from operational truth. A cystoscope factory that documents real implementation, closes CAPAs, controls suppliers, and improves continuously will protect patients and budgets. Selecting such cystoscope manufacturers turns procurement into a resilient, data-driven partnership—exactly what a high-reliability cystoscope supplier should deliver.
A reliable cystoscope factory should hold ISO 13485, FDA registration, and CE/MDR compliance. These certifications confirm that the manufacturer follows internationally recognized quality management systems for medical devices.
Leading cystoscope manufacturers use validated processes (IQ/OQ/PQ), statistical process control, and automated leak testing. Each batch undergoes final quality checks to guarantee consistent optical clarity, bending performance, and patient safety.
Yes. A responsible cystoscope supplier maintains Corrective and Preventive Action (CAPA) logs that document root cause analysis, corrective actions, preventive steps, and closure verification for each nonconformity.
Hospitals should request a demonstration where the cystoscope factory retrieves the full genealogy of a random device, including raw materials, operator IDs, equipment used, and inspection results. This proves effective traceability and UDI readiness.
Trusted cystoscope manufacturers conduct supplier audits, enforce incoming quality inspections with defined AQLs, and maintain performance scorecards. Dual-sourcing critical components like image sensors also reduces procurement risk.
A capable cystoscope supplier provides technical documentation, risk management files, clinical evaluation reports, and post-market surveillance data. These documents help hospitals demonstrate compliance during regulatory inspections.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS