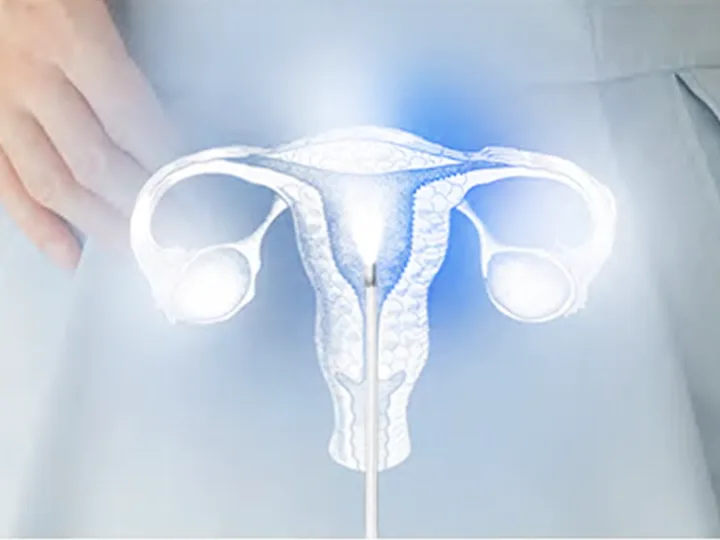
One-Stop Endoscope OEM Solutions for Rapid Market Success
We provide full-range OEM solutions for rigid, flexible, and disposable endoscopes. Backed by over 10 years of experience and a dedicated team in optics, precision machining, and medical imaging, we turn your ideas into high-performance, market-ready products. From multi-specialty adaptation to advanced optical modules and ergonomic design, we tailor every detail to meet clinical needs. Trusted by 150+ medical brands globally, we help partners stand out with innovative, cost-effective, and high-value endoscope solutions.
-
Diversified product design
• Support the whole process design from concept to finished product, or optimize based on the customer's existing solution
• Provide 2D/3D industrial design, ergonomic adaptation and appearance customization (material/color/logo) -
Multi-department adaptation development
• Covering the needs of urology, gynecology, gastroenterology and other specialties, customized with different diameters, lengths and viewing angles
• Special scene design (such as single-use, high temperature and high pressure sterilization resistance, etc.) -
Diversified optical solutions
• Customizable optical modules such as HD/4K imaging, fluorescence navigation, spectroscopic staining (such as NBI)
• Provides a variety of light source interfaces (LED/laser) and image processing algorithms (AI-assisted diagnosis) -
Functional expansion
• Integrated biopsy channel, flushing and suction, electrosurgical cutting and other functional modules
• Support wireless transmission, cloud storage or compatibility with third-party devices -
Material and process certification
• Medical grade stainless steel, titanium alloy or polymer materials are available, in compliance with ISO 13485/CE/FDA standards
• Precision machining (CNC/laser welding) ensures durability and sealing -
Capacity and delivery guarantee
• Modular production lines support small-batch trial production to large-scale delivery
• Provide global logistics and localized warehousing and distribution solutions -

Full compliance support
• Assist in completing registration inspections (biocompatibility, EMC, etc.), clinical evaluations and certifications in various countries (such as FDA 510k, MDR)
• Provide complete technical documentation (DHF/DMR) -

After-sales and iterative services
• Lifelong maintenance + technical upgrade support
• Jointly develop iterative products and share technical patents