Table of Contents
An XBX bronchoscope equipment system is designed to deliver clear imaging, smooth maneuverability, and dependable performance in pulmonary diagnostics and interventions. Engineered under ISO 13485, CE, and FDA standards, the XBX bronchoscope line integrates optical precision, ergonomic handling, and sterilization durability. Hospitals worldwide rely on it for consistent bronchoscopy outcomes and reduced total maintenance costs.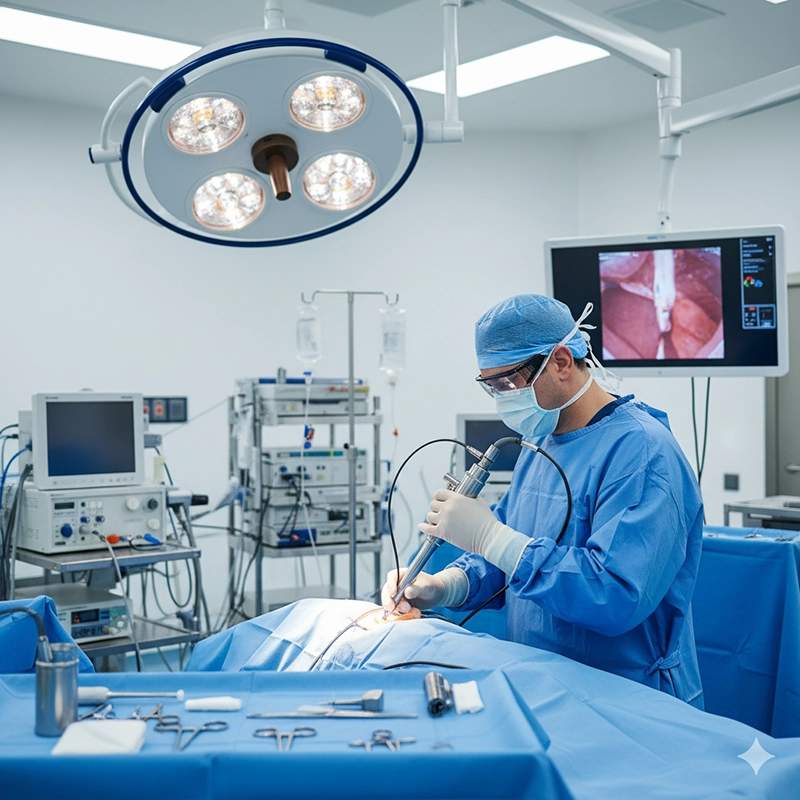
The design of the XBX bronchoscope equipment emphasizes clarity, flexibility, and infection control. Each component—from the distal lens to the control section—is engineered to provide accurate navigation through the airways while maintaining patient comfort and procedural safety. Compared with generic products, XBX uses premium-grade materials and digital process validation to achieve long-term reliability in demanding hospital environments.
High-resolution CMOS sensors deliver sharp images of airway walls and bronchial structures, improving lesion detection accuracy.
Illumination fibers are arranged for uniform brightness with minimal shadowing, allowing physicians to maintain clear visibility even in peripheral bronchi.
Lens coatings resist fogging and water droplet accumulation, reducing procedure interruptions.
The XBX bronchoscope equipment features lightweight construction and an intuitive control handle for precise tip movement. Smooth angulation, reinforced bending sections, and balanced torque response allow operators to navigate complex airways comfortably. The design minimizes hand fatigue, which is critical during prolonged procedures such as therapeutic bronchoscopy or endobronchial biopsy.
All patient-contact materials meet ISO 10993 biocompatibility standards and have been validated for repeated sterilization cycles.
Channels are constructed with seamless internal linings to reduce bacterial adhesion and simplify cleaning.
Leak tests and pressure integrity checks are conducted on each unit before shipment to prevent cross-contamination risks.
Within the XBX factory, bronchoscope manufacturing combines precision engineering, automated inspection, and digital traceability. This ensures each bronchoscopy device leaving the production line meets the same high standard of optical performance and mechanical integrity. Ordinary manufacturers often rely on manual assembly, which introduces variability—XBX eliminates that through data-driven control.
Critical components such as insertion tubes and distal lenses are produced under SPC-controlled machining processes.
Automated optical inspection verifies alignment and resolution consistency across batches.
Torque and bending stiffness mapping ensure identical handling characteristics between units.
Stainless-steel coil structures and multi-layer polymer sheathing protect internal components from wear and deformation. Bending sections use fatigue-resistant alloys to maintain performance after thousands of articulation cycles. As a result, the XBX bronchoscope equipment demonstrates extended life compared with standard models, reducing replacement frequency and overall cost for hospitals.
Each bronchoscope is serialized and linked to complete inspection records, allowing hospitals to access calibration and maintenance data easily.
UDI (Unique Device Identification) compliance enables tracking through global hospital asset systems.
Comprehensive documentation supports procurement transparency and regulatory audits.
Clinicians using XBX bronchoscopy equipment benefit from superior imaging precision and smooth workflow integration. The combination of optical clarity, reliable angulation, and intuitive controls enhances procedural efficiency in diagnostic and therapeutic bronchoscopy alike.
4K-compatible output improves visualization of mucosal lesions and vascular patterns.
Enhanced depth perception aids in biopsy site targeting and tool guidance.
Image color consistency supports accurate tissue differentiation under variable lighting.
The XBX bronchoscope machine accommodates accessories such as biopsy forceps, cytology brushes, and cryoprobes. Smooth channel transitions reduce friction, allowing quick instrument exchanges during interventional bronchoscopy. This reduces procedural time and improves precision during airway clearance or tumor removal procedures.
Plug-and-play integration with XBX endoscopy systems ensures rapid setup in operating rooms and ICUs.
Auto-calibration features reduce preparation time, helping clinicians focus on patient care.
Compact design simplifies storage and transportation, making it suitable for both large hospitals and mobile clinics.
XBX offers both reusable and single-use bronchoscope solutions. Disposable bronchoscope models are ideal for infection-sensitive environments such as ICUs or emergency departments. They provide the same image quality while eliminating cross-contamination risks and reprocessing costs, offering flexibility for hospital administrators managing diverse procedural needs.
Every XBX bronchoscope undergoes rigorous performance testing before shipment. The testing regime replicates real clinical stresses, ensuring that mechanical, optical, and electronic functions remain stable throughout their service life.
Resolution, distortion, and color accuracy are verified against calibrated test charts.
Light intensity and fiber transmission efficiency are measured for uniform output.
Image processors are synchronized to ensure zero-latency video transmission.
Each bronchoscope is bent through full angulation cycles thousands of times to verify durability.
Insertion tubes are tested for tensile strength and compression resistance.
Control handles are evaluated for torque consistency and ergonomic stability under extended use.
Helium leak tests confirm channel integrity after thermal and pressure stress.
Repeated sterilization cycles simulate years of clinical use without degradation of seals or optics.
Hydrophobic filters and valve systems are tested for airflow safety and bacterial barrier performance.
Leakage current and insulation resistance tests guarantee patient and operator safety.
EMC testing ensures the bronchoscope system operates reliably alongside other surgical equipment.
Grounding continuity and thermal monitoring systems meet IEC 60601-1 requirements.
Hospitals that adopt XBX bronchoscopy equipment benefit from a comprehensive package of technology, safety, and service reliability. It provides higher uptime, better imaging accuracy, and predictable maintenance schedules, making it a cost-efficient investment for pulmonary care units and surgical departments.
Lower lifetime cost due to extended product life and reduced failure rate.
Improved procedure turnaround time through easy setup and minimal maintenance requirements.
Compatibility across multiple imaging systems, allowing shared use within endoscopy departments.
Sharper imaging supports early diagnosis of airway lesions and infections.
Reduced patient discomfort from smoother insertion and shorter procedure duration.
Stable imaging and fluid management reduce complications during bronchoscopy.
XBX bronchoscope equipment is trusted by hospitals across continents for its consistent performance, strong service network, and transparent manufacturing documentation. Medical teams appreciate XBX’s responsive technical support, which ensures operational continuity even in high-demand pulmonary units. Through continuous innovation and clinical feedback, XBX maintains leadership in bronchoscopy technology.
The XBX bronchoscope equipment exemplifies how precision engineering and safety-driven design can transform pulmonary diagnostics. By combining advanced imaging, ergonomic handling, and long-term durability, XBX offers hospitals a dependable solution for bronchoscopy procedures that demand accuracy and reliability. This focus on consistency, safety, and clinical value defines why XBX remains a preferred name in modern respiratory care.
XBX bronchoscope equipment integrates high-resolution CMOS imaging, smooth articulation, and ISO 13485-certified manufacturing. These elements ensure clear visibility, consistent torque response, and exceptional reliability compared with generic bronchoscopes.
Each bronchoscope undergoes helium leak testing, insulation verification, and biocompatibility validation. The manufacturing process adheres to ISO 13485, CE, and FDA standards to guarantee electrical and biological safety for both patients and medical staff.
Yes. The XBX bronchoscope connects seamlessly to most existing hospital endoscopy systems and processors. Its plug-and-play design allows clinicians to integrate it without modifying workflow or infrastructure.
Yes. XBX provides single-use bronchoscopes ideal for ICUs and emergency rooms, where infection control and quick turnaround are critical. Disposable models maintain the same imaging quality as reusable ones while eliminating reprocessing time.
With proper maintenance, XBX reusable bronchoscopes can exceed 1,000 articulation and sterilization cycles without degradation in optical or mechanical performance, outperforming standard devices in lifespan and stability.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS