Table of Contents
The XBX Bronchoscope Factory delivers reliable OEM endoscopy systems by combining precision manufacturing, rigorous quality control, and advanced imaging technology under one integrated facility. Every bronchoscope produced by XBX undergoes optical calibration, sterilization validation, and functional testing to ensure hospitals receive consistent, ready-to-use devices. In short, reliability at XBX is not an afterthought—it’s the product of discipline, experience, and engineering integrity built into every stage of manufacturing.
So yes, when a hospital or distributor partners with XBX, they’re not just sourcing an instrument—they’re investing in a process refined by years of medical innovation. Let’s take a closer look at how that process unfolds behind the factory doors.
Decades ago, bronchoscopes were handcrafted devices—fragile, costly, and inconsistent. XBX entered the industry with a different vision: to industrialize precision without compromising safety. Located in a medical manufacturing zone equipped with ISO-13485 and CE-certified facilities, the XBX Bronchoscope Factory operates as both a research center and a production hub.
2008: Establishment of optical R&D division specializing in medical imaging lenses.
2014: Launch of flexible bronchoscope assembly lines with automated welding and leak testing.
2020: Integration of AI-based inspection for light fiber alignment.
2024: Expansion to OEM/ODM cooperation with hospitals and global distributors.
Every upgrade reflects one purpose: turning precision engineering into consistent clinical outcomes.
Walking through the XBX factory feels more like entering a laboratory than a workshop. Cleanrooms hum quietly as technicians assemble fiber bundles under microscopes. Automated robots handle lens coating and alignment while human engineers perform the delicate calibration that machines cannot replace.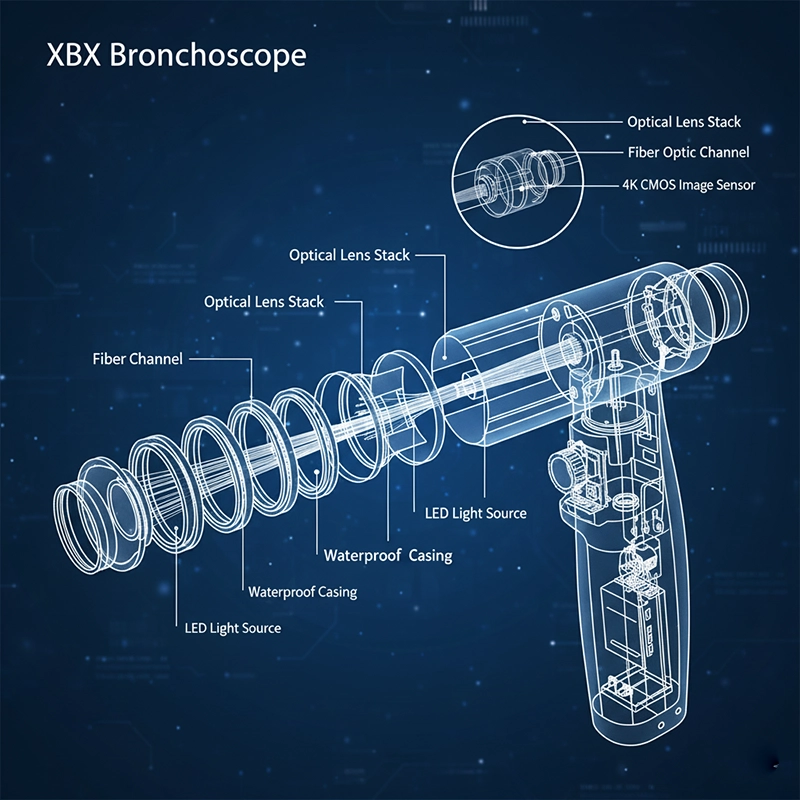
Optical fabrication: Multi-layer anti-reflection coating ensures maximum light transmission and accurate color rendering.
Insertion tube assembly: High-grade polymer sheath enhances flexibility without image distortion.
Image sensor integration: HD CMOS sensors provide consistent brightness even in narrow bronchi.
Leak and durability testing: Each unit is pressure-tested to withstand sterilization and repeated use.
Final sterilization validation: Ethylene oxide and plasma sterilization confirm patient safety.
So yes, precision at XBX isn’t theoretical—it’s visible in every layer of glass, steel, and light fiber.
Reliability starts with measurement. Every bronchoscope produced at the XBX factory passes through a strict, data-driven inspection protocol. Instead of relying solely on random sampling, the facility employs full-cycle verification—tracking each scope’s optical performance, bending angle, and suction channel integrity through a digital database.
Incoming material inspection (optical fiber, stainless steel, connectors).
Process control during assembly with automated optical testing.
Intermediate leak and deflection angle tests for mechanical stability.
Final performance verification using live bronchoscopy simulation.
Post-sterilization audit before packaging and labeling.
The reason is simple: consistency creates confidence. That’s why XBX maintains less than 0.3% return rate worldwide.
One of XBX’s strengths is its ability to adapt production for hospital systems and medical distributors through OEM and ODM services. Clients can request specific optical diameters, working channel sizes, or handle designs to match their procedural protocols. The engineering team uses CAD modeling and rapid prototyping to validate each design before full production.
Private-label branding and laser engraving.
Custom handle ergonomics for left- or right-handed surgeons.
Integration with proprietary imaging towers or processors.
Alternative sterilization compatibility (ETO, autoclave, plasma).
Color-coded tubing and connectors for multi-department identification.
So yes, whether you’re a hospital procurement officer or a distributor building your own brand, XBX provides a manufacturing backbone that makes it possible.
A major hospital group in Germany sought a bronchoscope line optimized for intensive-care use. Their priorities were image stability, quick sterilization, and ergonomic grip. XBX engineers collaborated remotely, adjusted the control section angle, and modified the suction valve for one-handed operation. After a six-month trial across five hospitals, the network reported a 28% reduction in procedure time and higher clinician satisfaction scores.
Dr. Ulrich Meyer, the project lead, summarized the partnership: “We were impressed not just by product quality but by how quickly XBX responded to feedback. They built, tested, and improved each iteration like partners, not suppliers.”
That’s precisely what differentiates XBX in the OEM market—responsiveness grounded in engineering discipline.
Beyond production, XBX invests heavily in R&D to refine endoscopic visualization. Its latest flexible bronchoscope integrates adaptive white-balance correction and low-noise image amplification for improved visualization in pediatric airways. Engineers are also exploring AI-assisted navigation to help physicians trace bronchial paths automatically.
4K sensor module for superior brightness and depth perception.
Hydrophobic lens coating preventing fog during extended use.
Smart lighting adjustment responding to tissue color contrast.
Digital recording interface for telemedicine and education.
In short, innovation at XBX doesn’t chase trends—it answers clinical challenges directly from the operating room.
Sustainability in medical device production is no longer optional. XBX has implemented waste-reduction programs and eco-friendly packaging across its factory. The company also adheres to fair-labor policies and transparent supplier audits. All materials are traceable and compliant with RoHS and REACH standards, ensuring global distribution readiness.
By combining responsible sourcing with technological precision, XBX demonstrates that reliability extends beyond performance—it includes ethics and sustainability.
Feedback from hospitals using XBX bronchoscopes consistently points to ease of handling, image clarity, and durability. Respiratory departments report fewer lens fog issues and smoother suction flow compared with competing models.
“We performed over 400 bronchoscopies last year with XBX systems and had zero mechanical failures.” — Head Nurse, Singapore General Hospital.
“The image fidelity allows us to detect subtle mucosal changes that standard scopes often miss.” — Pulmonologist, Seoul National University Hospital.
“Maintenance is straightforward. The modular handle saves us hours in servicing.” — Biomedical Engineer, London Healthcare Group.
So yes, the reputation of XBX isn’t built on claims—it’s written in clinical results.
For medical distributors, reliability equals market confidence. The XBX factory simplifies the procurement process through transparent pricing, consistent lead times, and multilingual after-sales support. OEM partners receive detailed product documentation, CE and FDA certification copies, and direct engineer contact for technical queries.
Flexible MOQ for pilot programs and tenders.
Fast delivery from global logistics hubs.
Dedicated OEM manager for communication and customization.
Marketing collateral support and training videos.
When distributors carry XBX, they carry credibility—the kind that keeps customers coming back.
Looking ahead, XBX aims to expand its bronchoscope portfolio into single-use and hybrid designs to meet infection-control demands. Integration with cloud-based imaging platforms will allow surgeons to store and review procedures securely. Research is also underway on carbon-neutral production methods and recyclable device components.
As global healthcare shifts toward precision and sustainability, the XBX Bronchoscope Factory continues to evolve as both manufacturer and innovator—proving that reliability isn’t a slogan, but a measurable standard.
In the end, the story of XBX is simple: engineering precision, ethical manufacturing, and lasting trust—one bronchoscope at a time.
The XBX Bronchoscope Factory focuses on designing and manufacturing high-quality bronchoscopes and OEM endoscopy systems. Each product is developed with strict optical calibration, leak testing, and sterilization validation to meet hospital-grade safety and imaging standards.
Every bronchoscope produced at the XBX factory undergoes a five-stage quality control process, including optical testing, mechanical durability checks, and real-use bronchoscopy simulation. Each unit is digitally tracked to ensure performance consistency and traceability from assembly to shipment.
XBX provides full OEM and ODM customization, allowing partners to modify scope diameter, handle design, imaging sensor type, and branding. Hospitals can request configurations compatible with their existing imaging towers, ensuring seamless integration and reduced training time.
XBX combines reliability with flexibility. Distributors benefit from low minimum order quantities, transparent production timelines, and multilingual technical support. Each shipment includes CE, ISO, and FDA documentation, making regulatory clearance smooth for global partners.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS