Table of Contents
An XBX hysteroscopy machine is engineered to deliver advanced visualization and precision during gynecologic diagnostics and surgical procedures. Designed under ISO 13485 and CE-certified manufacturing, every XBX hysteroscope integrates high-definition optics, fluid management, and ergonomic control to help hospitals achieve accurate uterine assessment and efficient workflow while minimizing patient discomfort.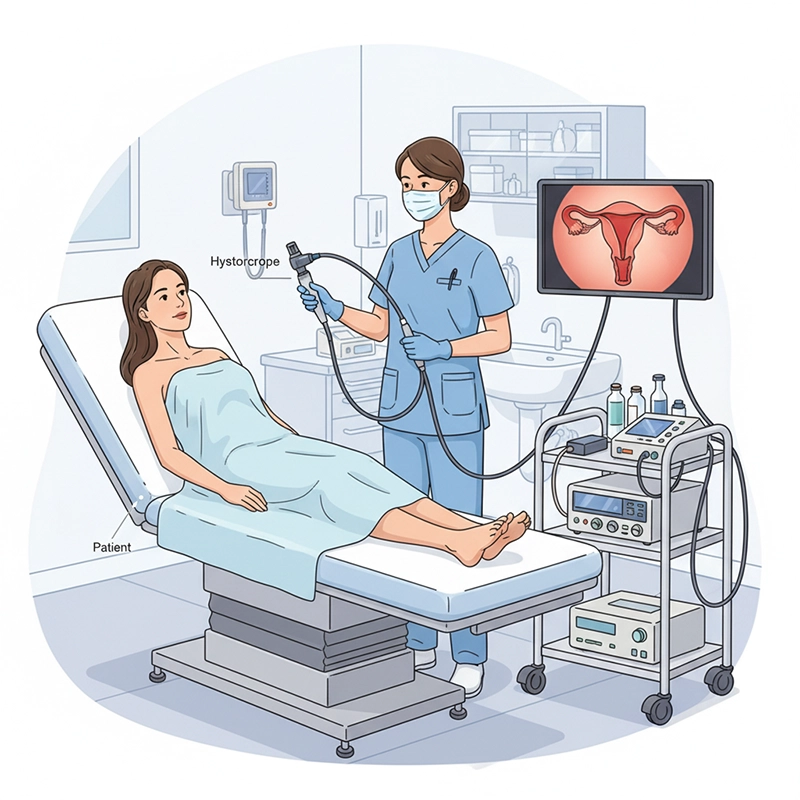
The XBX hysteroscopy machine integrates optical, electronic, and fluidic modules within a tightly controlled production framework. The goal is to deliver clear, distortion-free imaging inside the uterine cavity even under challenging visibility conditions. Each hysteroscope is calibrated with advanced optical benches to ensure accurate color rendition and depth perception that physicians rely on during hysteroscopic examinations.
Multi-element lenses are aligned using micron-level fixtures to maintain focus uniformity across the field of view.
Anti-reflective coatings and sealed distal windows are applied to prevent glare and fogging during procedures.
Every hysteroscope passes through modulation transfer function (MTF) testing to verify imaging resolution and contrast ratio.
The hysteroscopy machine employs a digital endoscope camera linked to a medical-grade processor. The XBX 4K imaging platform enhances visibility of intrauterine tissue structures, supporting accurate identification of fibroids, adhesions, and endometrial polyps. Optical fiber illumination has been optimized to maintain constant brightness, while the LED light source in the endoscopy equipment is tuned for color temperature stability to improve tissue differentiation.
To maintain a stable uterine environment, the XBX hysteroscopy machine uses an intelligent fluid control system. Inflow and outflow are balanced automatically through sensors that monitor pressure and flow rate. Compared with conventional devices, XBX systems provide superior cavity distension stability, which leads to a clearer surgical field and lower risk of fluid overload complications.
Hospitals adopting XBX hysteroscopy equipment experience shorter setup times and better user ergonomics. Cable routing, connector design, and touch-screen control panels are simplified for efficient operation. Modular assembly allows the same imaging console to connect with hysteroscope, cystoscope, or laparoscope components, reducing inventory and maintenance costs for hospitals and distributors.
The reliability of a hysteroscopy machine directly influences patient safety and procedural outcome. XBX has implemented rigorous validation protocols to ensure each system meets medical safety standards, electrical insulation requirements, and biocompatibility regulations. Ordinary devices often fail due to inconsistent sealing or image drift; XBX systems are protected by advanced mechanical design and durability verification under accelerated life testing.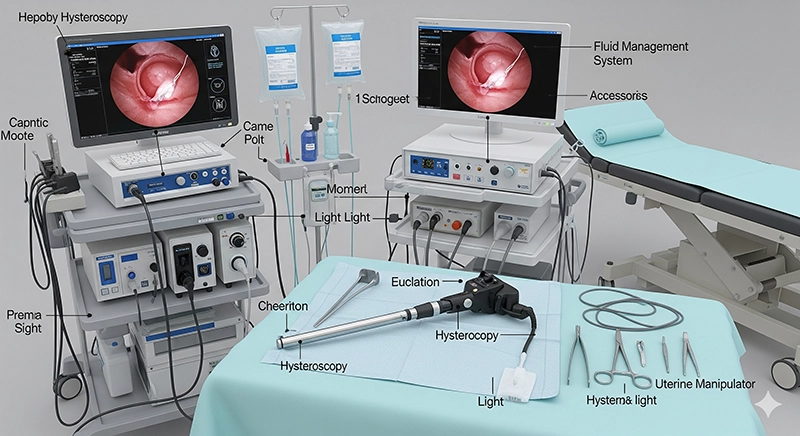
Medical-grade stainless steel and high-strength polymer housings are used for corrosion resistance and sterilization stability.
Optical adhesives and sealing materials are validated through repeated autoclave and chemical exposure cycles.
Every patient-contact component complies with ISO 10993 biocompatibility requirements and FDA material safety standards.
Each hysteroscope undergoes articulation fatigue testing, thermal cycling, and leak inspection. The bending section and insertion shaft are designed to withstand thousands of reprocessing cycles without affecting imaging alignment. Data collected from life testing supports hospitals’ procurement teams in estimating real operational lifespan, a major factor in long-term cost control.
All systems pass electrical leakage, grounding, and insulation resistance testing to conform with IEC 60601 standards.
Power supplies feature medical-grade isolation to prevent cross-device interference in the operating room.
Safety firmware monitors internal temperature and power draw, automatically shutting down in abnormal conditions.
XBX applies ISO 14971 risk control at every production stage. Device history records (DHR) include component traceability, calibration logs, and test results. This transparency provides hospitals and distributors confidence in regulatory compliance and device authenticity.
Ordinary hysteroscopy systems often rely on analog imaging and manual fluid control, leading to inconsistent visualization and operator fatigue. The XBX hysteroscopy machine eliminates these weaknesses by merging digital 4K visualization, intelligent pump systems, and ergonomic design. As a result, gynecologists can work faster, identify pathology more clearly, and perform interventions with higher confidence.
4K video endoscope sensors deliver detailed imaging that enhances visualization of endometrial microstructures.
Color and light uniformity are preserved across procedures due to consistent calibration of the imaging system.
Real-time recording and image capture functions enable traceable case documentation for hospital quality systems.
XBX hysteroscopy equipment is built to minimize downtime. Components can be replaced independently, and preventive maintenance schedules are guided by sensor feedback data. The modular console allows one imaging processor to serve multiple surgical specialties, providing long-term flexibility for hospital investment planning.
Image resolution: Ordinary systems use HD sensors; XBX adopts 4K imaging for higher diagnostic accuracy.
Fluid control: Manual pressure regulation is replaced with intelligent automatic balancing.
Durability: Standard scopes last fewer than 500 cycles; XBX units sustain over 1,000 reprocessing cycles.
Service structure: XBX provides global maintenance centers and traceable calibration records for every unit.
The XBX hysteroscopy machine supports DICOM and network connectivity, allowing seamless integration with hospital EMR and PACS systems. Procedure videos and still images can be archived directly, supporting data-driven patient management and research.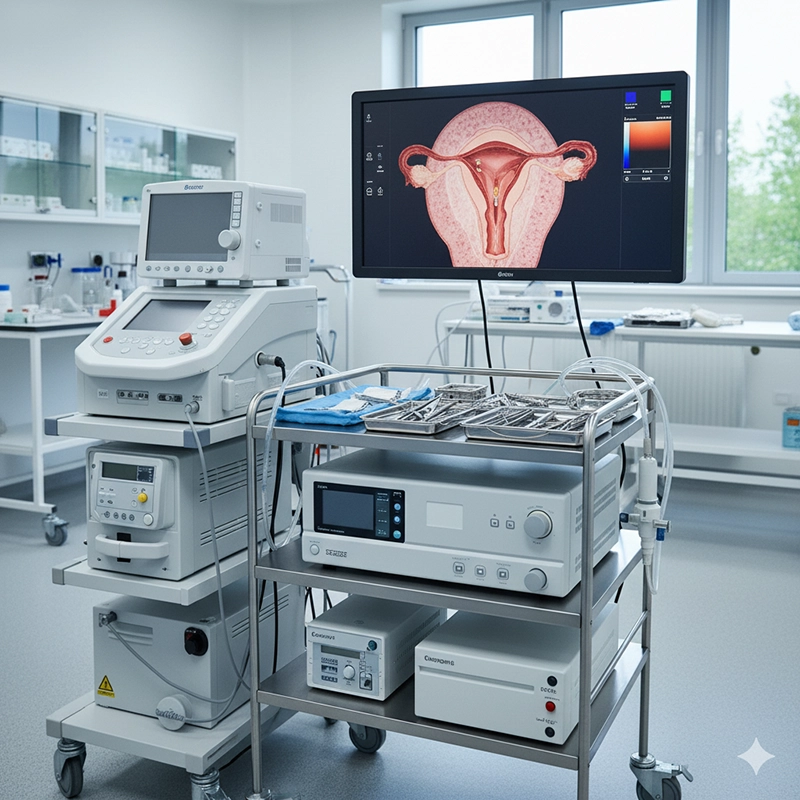
Each hysteroscopy machine is validated under simulated hospital conditions to ensure performance reliability and safety. Testing covers mechanical, optical, and electronic functions to verify that the equipment can perform consistently across different clinical environments.
Automated lens calibration ensures consistent focus accuracy within 0.01 mm tolerance.
Distortion mapping and chromatic correction are conducted to guarantee clear imaging at all zoom levels.
Optical pathways are sealed to prevent dust contamination that may affect clinical performance.
Pressure sensors are calibrated to ±1 mmHg for accurate intrauterine pressure management.
Flow rates are verified to maintain stable cavity expansion with minimal fluid loss.
System alarms are validated to warn operators of fluid imbalance or tubing blockage.
Helium and submersion leak tests are applied to every unit before shipment.
Articulation joints and seals are stress-tested under cyclic loading to ensure long-term endurance.
Packaging undergoes vibration and drop testing to protect the hysteroscope during transportation.
Surge and ESD tests confirm resistance to voltage fluctuations and static interference.
Shielding materials minimize electromagnetic noise, ensuring stable video transmission.
All units pass burn-in testing under continuous operation for 72 hours before release.
For hospitals, the XBX hysteroscopy machine represents not only a technical upgrade but also an operational advantage. Its reliability, low maintenance costs, and multi-specialty compatibility make it a preferred choice for modern gynecologic departments. For patients, higher imaging precision and improved comfort lead to safer, faster, and more accurate procedures.
Lower maintenance and operating costs through modular design and extended device life.
Faster setup and procedure turnaround with integrated image capture and fluid control.
Enhanced compliance with documentation and traceability requirements for accreditation audits.
Shorter examination time and less discomfort due to improved instrument control and visualization.
Reduced need for repeat procedures through clearer imaging and accurate diagnosis.
Lower infection risk from fully sterilizable materials and validated reprocessing cycles.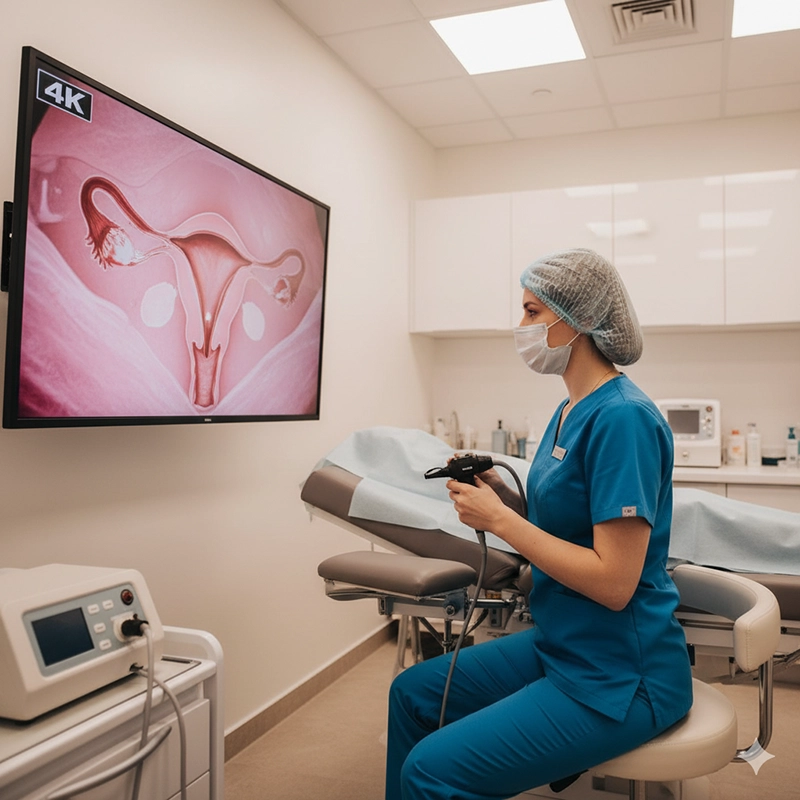
XBX hysteroscopy equipment has been deployed across teaching hospitals and private clinics worldwide. The brand’s emphasis on clinical reliability, user training, and service responsiveness has made it a trusted supplier in gynecologic endoscopy. As global standards evolve, XBX continues to innovate, ensuring its hysteroscopy machines remain ahead in precision imaging and operational efficiency.
The XBX hysteroscopy machine embodies the fusion of imaging science, safety engineering, and ergonomic design. By focusing on clarity, durability, and system integration, XBX has created a platform that supports hospitals in achieving better diagnostic outcomes and patient satisfaction. This commitment to precision and reliability defines why XBX continues to lead in modern hysteroscopic technology.
The XBX hysteroscopy machine delivers high-resolution 4K imaging with intelligent fluid control, allowing physicians to visualize the uterine cavity with exceptional clarity. This combination reduces diagnostic errors and shortens operation times compared to conventional hysteroscopic systems.
Each unit is manufactured under ISO 13485 and CE-certified standards. It undergoes optical calibration, electrical safety checks, and biocompatibility testing to guarantee long-term stability and compliance with hospital safety regulations.
Yes. The hysteroscopy machine connects seamlessly with most standard endoscopy processors and light sources. XBX also provides integration documentation to help hospitals maintain existing workflows while upgrading their imaging quality.
XBX offers modular spare parts, maintenance kits, and certified service centers. Preventive maintenance schedules are provided with sensor-guided alerts to reduce downtime and ensure consistent performance across all devices.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS