Table of Contents
Medical devices custom solutions offered by OEM endoscope manufacturers help hospitals, clinics, and distributors obtain tailored devices that meet specific clinical needs. By combining customized design, bulk procurement, and compliance with international standards, buyers can reduce costs and secure reliable supply chains. For procurement managers, understanding how OEM and ODM solutions work is essential to making informed decisions when sourcing endoscopes from factories worldwide.
Medical devices custom solutions refer to tailored equipment designed and produced according to the specific needs of healthcare providers, distributors, and research institutions. Unlike off-the-shelf products, custom solutions allow buyers to specify device dimensions, imaging quality, materials, and functional modules.
Endoscopes are one of the most requested medical devices for customization. Hospitals may require flexible scopes with ultra-thin diameters for pediatric applications, or rigid scopes with specialized accessories for surgical procedures. Distributors may want ODM services to launch their own private-label brand, sourcing endoscopes directly from manufacturers.
Key differences between standard and custom medical devices:
Standard devices: Pre-designed, mass-produced, limited flexibility.
Custom devices: Adjusted specifications, adaptable features, OEM/ODM production models.
As healthcare evolves, hospitals and procurement teams increasingly demand tailored medical solutions, which makes OEM endoscope manufacturers valuable partners.
OEM endoscope manufacturers are factories that design, develop, and mass-produce devices according to buyer specifications. They are not simply suppliers; they operate as strategic partners in the medical supply chain.
Under the OEM model, manufacturers produce endoscopes based on the design provided by the buyer. Hospitals and distributors benefit by reducing the need for in-house R&D while still obtaining high-quality products.
In the ODM model, factories provide their own ready-made designs, which can then be rebranded by buyers. This approach is especially valuable for distributors looking to expand into new markets with minimal development cost.
Access to advanced manufacturing technology
Lower entry barriers for custom product lines
Stronger supplier–buyer partnerships
Flexibility in branding and distribution
Diameter and Length: Pediatric vs adult endoscopes
Imaging Resolution: HD or 4K cameras
Working Channels: Single or multiple channels for instruments
Accessories: Biopsy forceps, light guides, suction tools
Per-unit cost reductions through volume pricing
Long-term contracts that ensure steady supply
Shorter lead times by purchasing directly from the endoscope factory
ODM private-label branding without new production lines
Faster time-to-market for distributors
Improved margins via direct factory cooperation
Production Capacity: Ability to handle bulk orders efficiently
R&D Strength: Integration of optics, electronics, and digital imaging
Quality Assurance: ISO 13485-certified production facilities
MOQ (Minimum Order Quantity): Commonly 50–500 units by product type
Lead Time: Clear scheduling for samples, pilot, mass production
After-Sales: Technical training, warranty, spare parts availability
CE Mark for European markets
FDA 510(k) for the United States
ISO 13485 for medical device quality systems
Local registrations for destination countries
Use side-by-side comparisons to evaluate which partner best matches your strategy—volume, cost, customization, or speed.
| Manufacturer Type | Strengths | Weaknesses | Best For |
|---|---|---|---|
| Large OEM Factory | High capacity, strict QC, global certifications | Higher MOQ, less flexible for small buyers | Hospitals, major distributors |
| Medium-Sized Factory | Balanced cost/customization, flexible MOQ | Limited global service network | Regional distributors |
| ODM Supplier | Ready-made designs, quick branding | Less design flexibility | Private-label distributors |
| Local Distributor | Fast delivery, easy communication | Higher cost, no factory control | Urgent, small-scale orders |

Asia: China, South Korea, and Japan lead in capacity and cost efficiency
Europe: Demand for CE-certified, high-end flexible endoscopes
North America: Preference for FDA-cleared devices and advanced imaging systems
Industry analyses project steady growth of the global OEM/ODM medical device market toward the late 2020s, with endoscopy systems contributing a meaningful share due to rising minimally invasive procedures and hospital modernization.
Define exact clinical specifications and use scenarios
Shortlist OEM endoscope manufacturers by capability and certification
Request samples and perform clinical or bench testing
Verify compliance documents (ISO, CE, FDA) and traceability
Negotiate bulk pricing, payment terms, and warranty scope
Agree on production schedule, acceptance criteria, and after-sales support
Certification Risk: Independently verify CE/FDA/ISO status
Contract Risk: Define responsibilities, IP, and liability clearly
Supply Chain Risk: Establish backup suppliers and safety stock
AI-Assisted Endoscopy: Decision support for lesion detection
Miniaturization: Pediatric and micro-endoscopy growth
Sustainability: Materials optimization and reusable designs
Remote Services: Digital training and global maintenance support
Hospitals will increasingly rely on OEM endoscope manufacturers not just for steady supply but also for innovation partnerships. Distributors will expand ODM brands into new markets with faster product launches and localized service.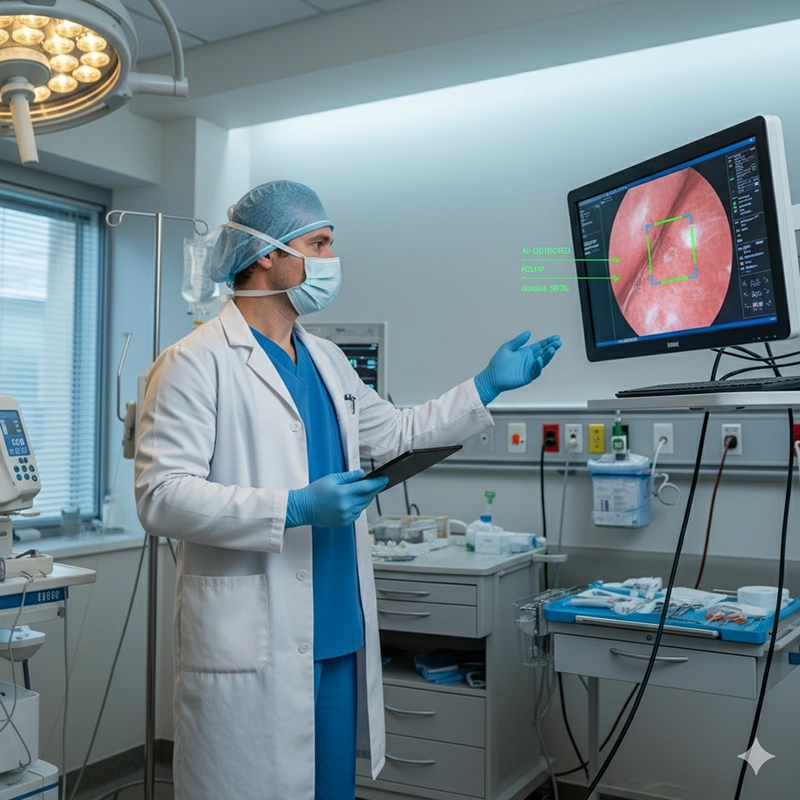
Medical devices custom solutions empower hospitals, clinics, and distributors to access tailored endoscopes that meet unique clinical and market needs. OEM endoscope manufacturers are central to building reliable supply chains, balancing cost and quality, and ensuring compliance across regions. For procurement managers, partnering with the right endoscope factory influences immediate budgets and long-term growth. As healthcare demand expands globally, OEM/ODM endoscope manufacturers will remain essential allies in delivering innovation, scale, and stability.
Yes. Our factory offers tailored solutions for flexible, rigid, and video endoscopes, including custom diameters, imaging quality, and accessory options to meet hospital and distributor requirements.
The minimum order quantity depends on the model. For standard custom designs, MOQ ranges from 50 to 100 units, while advanced or highly customized medical devices may require higher volumes.
Yes. ODM services are available for distributors who need ready-made designs rebranded under their own label, enabling faster market entry without additional R&D investment.
Yes. Sample units can be provided for testing clinical performance, image clarity, and durability before finalizing large-scale orders.
Each endoscope undergoes optical inspection, waterproof testing, sterilization validation, and electronic function checks under ISO-certified quality systems.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS