Table of Contents
Disposable medical endoscopes are redefining the global landscape of minimally invasive diagnostics. Hospitals across the world are increasingly adopting single-use devices to reduce infection risks, simplify reprocessing workflows, and align with new regulatory standards on patient safety. Yet, despite their rapid rise, reusable endoscopes remain indispensable for complex surgical procedures requiring high precision and image fidelity. Rather than a replacement, the current transformation represents a diversification of endoscopic technology, shaped by infection control, economic logic, environmental sustainability, and continuous innovation.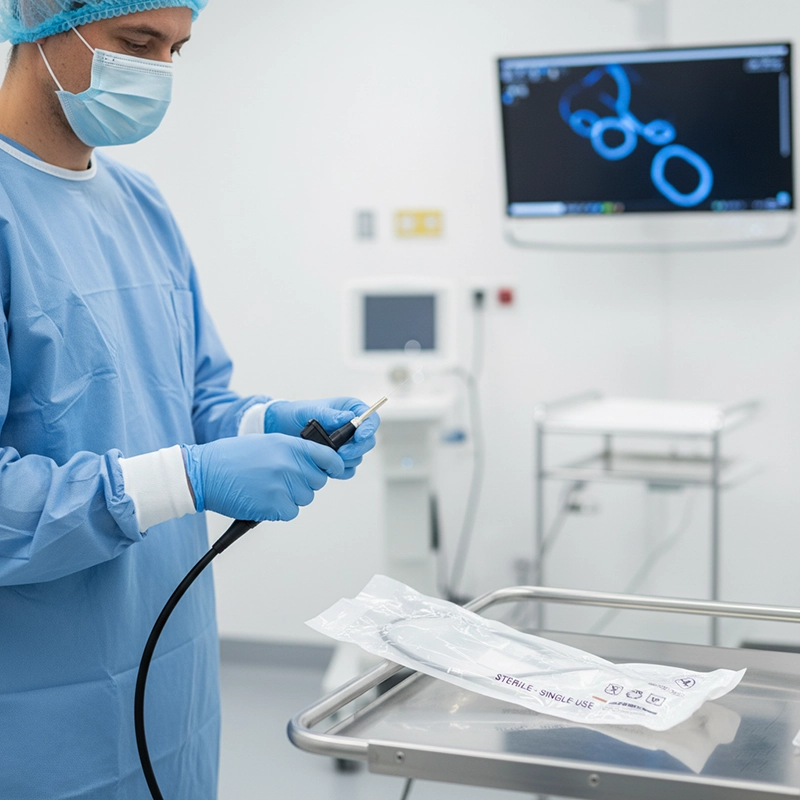
Over the past decade, disposable medical endoscopes have shifted from niche experimental devices to mainstream tools in critical care, pulmonology, and urology. Their emergence coincides with growing global awareness about hospital-acquired infections (HAIs) and biofilm contamination inside reusable scopes. The pandemic accelerated this shift: during COVID-19, disposable bronchoscopes became essential for safe airway management in intensive care units. This momentum continued post-pandemic, transforming temporary solutions into permanent protocols.
In 2025, single-use endoscopes account for approximately 20% of all flexible endoscopy procedures in high-income countries, compared to less than 5% in 2018. Hospitals cite multiple reasons for adoption: zero risk of cross-contamination, reduced sterilization overhead, and faster procedural turnover. For large healthcare systems, disposables provide logistical agility—especially where patient throughput is high, and reprocessing bottlenecks slow workflow efficiency.
| Region | Adoption Drivers | Market Share (2025 est.) |
|---|---|---|
| North America | Strict infection regulations, strong disposable supply chains | 30–35% |
| Europe | Environmental regulation balanced with infection control | 25% |
| Asia-Pacific | Cost-sensitive procurement, slower adoption pace | 10–15% |
| Latin America & Africa | Limited waste management infrastructure | Below 10% |
These figures reveal that replacement is not absolute but contextual. Wealthier systems transition faster due to stronger infection control mandates and liability concerns, while developing markets continue favoring reusable systems for cost efficiency.
Every technological shift in medicine begins with a crisis. The global transition toward disposable endoscopes began when numerous infection outbreaks were linked to inadequately cleaned reusable duodenoscopes. Despite sophisticated reprocessing machines and enzymatic detergents, internal microchannels often retained organic residue and bacteria. Studies by the FDA found that even after proper cleaning, up to 3% of reusable scopes still tested positive for pathogens. This unacceptable risk triggered a re-evaluation of traditional assumptions.
Disposable endoscopes remove the weakest link: human error. Each device arrives sterile, factory-sealed, and ready for use. After a single procedure, it is discarded. No reprocessing, no tracking logs, no risk of cross-patient contamination. Hospitals adopting disposables have reported significant drops in HAI rates—particularly in bronchial and urinary procedures where contamination risk is highest.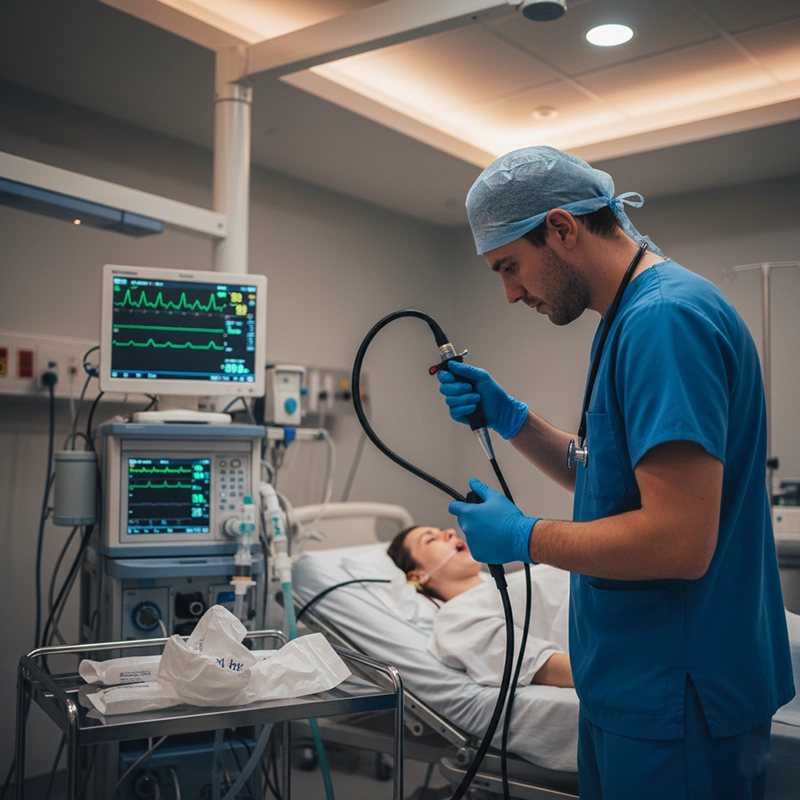
During the height of COVID-19, many hospitals replaced reusable bronchoscopes with disposable equivalents to protect staff and patients. At the University Hospital of Birmingham, disposable scope use reduced cross-infection risk by over 80% and allowed immediate post-procedure turnaround. Staff reported lower anxiety levels and faster workflow. Even after pandemic restrictions lifted, the hospital continued partial adoption as part of its infection-prevention strategy, demonstrating how temporary necessity evolved into lasting change.
At first glance, single-use endoscopes appear more expensive. A reusable scope costs approximately USD 40,000 and may last several years, whereas a disposable unit costs between USD 250–600 per procedure. However, direct comparison is misleading without considering the full cost of ownership, including maintenance, reprocessing labor, consumables, equipment downtime, and legal risk from infection incidents.
| Cost Factor | Reusable Endoscope | Disposable Endoscope |
|---|---|---|
| Initial Investment | High (USD 25,000–45,000) | None |
| Reprocessing per use | USD 150–300 | 0 |
| Maintenance / Repair | USD 5,000–8,000 annually | 0 |
| Infection Liability Risk | Moderate to high | Minimal |
| Per Procedure Cost (Total) | USD 200–400 | USD 250–600 |
When hospitals conduct risk-adjusted cost modeling, disposable scopes often yield lower “infection-adjusted cost per patient.” Smaller clinics benefit most—without large reprocessing departments, they avoid costly sterilization infrastructure and downtime. In tertiary hospitals, hybrid systems prevail: disposables are reserved for high-risk cases, while reusables handle routine or specialized interventions.
Improved operating room throughput due to zero cleaning time.
Lower insurance premiums through demonstrable infection control compliance.
Reduced staff burden and training time for reprocessing protocols.
Predictable per-case budgeting simplifies procurement cycles.
For administrators, this shift reframes disposable medical endoscopes not as consumables but as financial instruments optimizing safety and efficiency. Hospitals that quantify their hidden sterilization costs often discover that single-use devices offer better value than previously assumed.
The rise of disposables inevitably introduces environmental trade-offs. A typical single-use endoscope contains plastic housing, fiber optics, and electronic sensors—components not easily recyclable. When thousands are discarded monthly, environmental critics question whether improved infection safety justifies the ecological cost. Healthcare systems, under pressure from sustainability frameworks like the EU Green Deal, now demand greener product lifecycles.
Manufacturers are investing in biodegradable polymers and recyclable electronics to reduce carbon footprint. Some, including XBX, have introduced take-back programs that disassemble used scopes into recyclable metal and plastic parts. In pilot programs, up to 60% of non-contaminated components were successfully recovered and reused in non-clinical applications. Hospitals are also experimenting with “green procurement criteria,” requiring suppliers to submit sustainability certifications alongside ISO and CE compliance documents.
Environmental responsibility is becoming a competitive advantage. In tenders across Europe, hospitals increasingly favor vendors with eco-design initiatives. This trend is reshaping the market: the next generation of disposable endoscopes may no longer be fully disposable but “semi-circular,” incorporating reusable handles and replaceable distal sections. This evolution reduces waste volume by over 70%, bridging infection control and ecological stewardship.
The earliest single-use endoscopes were perceived as inferior substitutes—grainy images, limited articulation, and poor illumination. Today’s devices tell a different story. Advances in CMOS sensors and LED miniaturization have closed the quality gap dramatically. High-resolution disposable scopes now provide 1080p or even 4K imaging, rivaling reusable systems used in gastroenterology or ENT.
Real-time image transmission via Wi-Fi or USB-C interfaces.
Direct data archiving into hospital PACS systems.
Compatibility with AI-based lesion detection algorithms.
Onboard data encryption ensuring patient privacy.
Manufacturers like XBX have embraced this digital integration trend by offering modular imaging platforms: a reusable imaging processor paired with disposable scope attachments. The result is reduced per-use waste and superior image fidelity. Clinicians report that such systems combine the tactile familiarity of traditional scopes with the sterility benefits of single-use designs.
Artificial intelligence is emerging as the next frontier. Disposable scopes with integrated AI modules can detect abnormalities, track procedural metrics, and auto-generate reports. These capabilities transform the disposable device from a simple instrument into a data-driven diagnostic tool. Hospitals using AI-enabled scopes have reported reductions in documentation time by up to 40%, freeing clinicians to focus on patient interaction. In the long term, these technologies may reshape not only infection control but also clinical efficiency.
The transition from reusable to disposable medical endoscopes depends heavily on clinician confidence. Experienced surgeons develop tactile memory with reusable systems—weight distribution, torque response, and articulation feel. Early single-use devices felt foreign, lighter, and less stable. Manufacturers have since addressed these ergonomic issues by refining material stiffness and improving handle feedback. The latest XBX disposable scopes, for example, emulate reusable control dynamics so closely that transition time for experienced users is minimal.
In user studies across 12 hospitals, over 80% of physicians rated modern disposable scopes as “clinically equivalent” for diagnostic tasks. However, most agree that reusables retain advantages in advanced therapeutic interventions that require multiple accessory channels or continuous suction. The distinction is clear: disposables excel in accessibility and safety, while reusables dominate in procedural complexity. This complementary relationship defines the practical reality of modern endoscopy.
Regulatory frameworks now reinforce the momentum of disposable technologies. The FDA’s guidance encourages transition to single-use or partially disposable designs in response to repeated contamination incidents. In the European Union, the MDR (Medical Device Regulation) enforces stricter traceability for reusable instruments, indirectly favoring disposables due to simpler compliance. In Asia, governments incentivize local manufacturing of single-use devices to reduce dependency on imported reusables.
Risk-based purchasing models combining infection probability and environmental cost.
Vendor evaluation including ISO 13485, CE, FDA clearance, and sustainability scorecards.
Hybrid fleet management—reusable base systems with disposable modules.
OEM customization options for branding and regional supply resilience.
Hospital administrators increasingly treat endoscopy procurement as a strategic investment rather than routine equipment acquisition. Many adopt dual contracts: one supplier for reusable capital systems and another for disposable consumables. This diversification strengthens supply chain resilience and minimizes dependency on a single manufacturer. In this context, companies like XBX gain a competitive edge through OEM flexibility and consistent quality assurance.
Dr. Lin Chen, a hospital epidemiologist in Singapore, summarizes the shift succinctly: “Disposable endoscopes are not replacing reusables; they are replacing uncertainty.” The remark captures the psychological comfort disposables offer—complete assurance of sterility. Infection prevention teams embrace them not because they are cheaper or more advanced but because they eliminate the variable of human error.
Industry leaders echo this sentiment. Analysts from Frost & Sullivan forecast that by 2032, at least 40% of hospitals worldwide will employ a mixed-model endoscopy fleet. Hybridization, not replacement, defines the future trajectory. The medical ecosystem is learning to balance technology, economics, and ecology simultaneously—a triad that demands both innovation and restraint.
The disposable endoscope market has also transformed manufacturing logistics. Compared to reusables, which rely on precision optics and complex assembly, disposable scopes can be mass-produced with injection-molded components and printed circuitry. This scalability enables cost reduction and supply flexibility, supporting OEM contracts worldwide.
China has emerged as a major hub for disposable endoscope production, led by companies like XBX that combine ISO13485-certified facilities with international distribution networks. Europe remains the center for optical innovation, while North America drives regulatory and AI integration. The intercontinental collaboration between design, compliance, and manufacturing accelerates both quality and adoption speed.
Hospitals requesting private-label disposable scopes to align with procurement identity.
Regional distributors forming joint ventures with OEMs for supply stability.
Manufacturers offering end-to-end services—from mold design to regulatory filing.
Digital traceability systems linking batch IDs with sterilization logs.
The OEM/ODM flexibility makes disposable scopes particularly appealing to emerging healthcare systems. Instead of importing expensive reusable models, hospitals can source locally manufactured single-use devices that meet international standards, accelerating accessibility and healthcare equity across developing regions.
The long-term direction of the endoscopy industry is not binary. Disposable medical endoscopes will not eliminate reusable ones; instead, both will evolve in symbiosis. As technology advances, distinctions between them will blur—reusables will become easier to sterilize, and disposables more sustainable and high-performance. Hospitals will increasingly adopt “fit-for-purpose” policies: single-use for infection-sensitive or time-critical procedures, reusable for high-value, precision-dependent interventions.
By 2035, analysts predict a three-tier ecosystem:
Fully Disposable Tier: Simple diagnostic scopes, portable units for ICU and emergency use.
Hybrid Tier: Modular devices with reusable cores and disposable distal components.
Reusable Premium Tier: High-end systems for advanced surgical applications.
This layered model ensures both efficiency and sustainability. The success of this integration will depend on regulatory alignment, manufacturer transparency, and continued innovation in eco-materials and digital systems. In every scenario, the disposable medical endoscope stands as both a symbol and catalyst of a safer, smarter, and more adaptive medical future.
In the final analysis, disposables have not replaced reusables—they have redefined what hospitals expect from safety, flexibility, and responsibility. The future of endoscopy lies not in choosing one technology over another but in harmonizing both under a shared commitment to patient safety and sustainable progress.
Disposable medical endoscopes reduce infection risks by eliminating the need for reprocessing. Hospitals choose them for ICU, bronchoscopy, and urology cases where sterility is essential. Brands like XBX provide single-use solutions that balance safety, imaging quality, and cost predictability.
Per use, disposables may seem costlier, but they save money by avoiding sterilization labor, repairs, and infection-related liabilities. Economic studies show comparable total costs once hidden reprocessing expenses are included.
XBX single-use endoscopes integrate HD CMOS sensors and ergonomic control design, delivering clear imaging without cleaning steps. They offer wireless data transfer and meet CE and FDA standards, making them ideal for fast-paced hospital environments.
Unlikely. The market is evolving toward hybrid systems—reusable imaging cores with disposable distal ends. This approach combines high precision with infection safety. Reusable systems will remain vital for complex surgeries, while disposables dominate routine diagnostics.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS