Table of Contents
Hospital procurement strategies for colonoscope OEM ODM in 2025 represent a critical intersection between cost management, quality assurance, and technological advancement. As hospitals face growing demand for minimally invasive diagnostics and preventive healthcare, purchasing colonoscope equipment directly from OEM and ODM suppliers has emerged as a preferred strategy. This approach allows hospitals to customize colonoscopy machines and colonoscopy systems, optimize budgets, and establish long-term supplier relationships that ensure both product reliability and regulatory compliance. By carefully selecting colonoscope manufacturers, colonoscope factories, and colonoscope suppliers, hospitals can achieve operational efficiency while maintaining patient safety and clinical outcomes.
The adoption of colonoscope OEM and ODM procurement models has transformed hospital purchasing processes. Traditionally, hospitals relied on distributors to source colonoscopy equipment. However, direct engagement with colonoscope manufacturers and colonoscope factories reduces costs, increases transparency, and enhances opportunities for product customization. Hospitals today demand colonoscopy machines and colonoscopy systems that integrate seamlessly into digital workflows, provide high-definition imaging, and comply with international safety standards.
Cost efficiency: By sourcing directly from colonoscope factories, hospitals often save up to 30% compared to distributor pricing.
Customization: Colonoscope manufacturers can modify features such as imaging resolution, ergonomics, and sterilization compatibility based on hospital specifications.
Quality assurance: Reputable colonoscope suppliers ensure compliance with ISO13485, CE, and FDA certifications, guaranteeing safe clinical use.
After-sales service: OEM ODM agreements frequently include spare parts supply, training, and maintenance contracts that extend the lifecycle of colonoscopy equipment.
Selecting the right colonoscope supplier or colonoscope factory requires hospitals to evaluate multiple factors beyond price. While cost remains an essential criterion, issues such as supply chain resilience, product quality, and technical innovation strongly influence procurement decisions.
ISO13485 certification ensures quality management systems for medical devices.
CE and FDA approvals confirm safety and performance standards for colonoscopy machines.
Clinical trial data demonstrates reliability and diagnostic accuracy of colonoscopy systems in real-world hospital environments.
Hospitals must compare bulk procurement from colonoscope manufacturers against distributor-led purchasing models.
Total cost of ownership includes maintenance, accessories, and training costs for colonoscopy equipment.
ODM services provide additional value by enabling hospitals to introduce custom-branded colonoscope devices.
Colonoscope factories with global distribution networks provide reliable product availability.
Regional stock centers reduce lead times for urgent colonoscopy machine replacements.
Partnerships with multiple colonoscope suppliers protect against supply chain disruptions.
The colonoscope price landscape in 2025 is influenced by regional economics, technological innovations, and regulatory requirements. Hospitals evaluating colonoscopy equipment procurement must consider not only upfront colonoscope price but also long-term costs associated with service, sterilization, and upgrades. Recent market reports indicate that colonoscope manufacturers in Asia-Pacific lead in cost efficiency, while European and North American suppliers emphasize premium features and compliance.
| Region | Average Colonoscope Price (OEM) | Average Colonoscope Price (ODM) |
|---|---|---|
| North America | $3,500–$5,000 | $3,800–$5,500 |
| Europe | $3,000–$4,800 | $3,200–$5,200 |
| Asia-Pacific | $2,500–$4,200 | $2,700–$4,500 |
| Latin America | $2,800–$4,500 | $3,000–$4,700 |
Hospitals in Asia-Pacific benefit from competitive colonoscope factory production hubs, while higher regulatory costs in North America and Europe contribute to elevated colonoscope prices. For hospitals pursuing large-scale colonoscopy system procurement, OEM ODM partnerships remain the most cost-effective route.
Hospitals must adopt structured procurement strategies to maximize returns from colonoscope OEM ODM arrangements. This involves not only financial evaluations but also technical, logistical, and regulatory considerations. A procurement team that balances these factors can ensure that colonoscopy machines and systems meet both clinical and budgetary needs.
Conduct technical evaluations of colonoscopy equipment from multiple colonoscope manufacturers.
Request product samples and pilot testing before signing long-term supply agreements.
Compare colonoscope prices from various suppliers to assess total value.
Negotiate extended service contracts with colonoscope factories, including preventive maintenance.
Ensure compatibility of colonoscopy systems with existing hospital IT infrastructure.
Hospitals seeking colonoscopy machines and colonoscopy systems must identify the essential technical specifications that meet their patient care objectives. Factors such as image resolution, maneuverability, sterilization, and software integration all play significant roles in procurement decisions.
High-definition imaging sensors for enhanced polyp detection.
Lightweight insertion tubes to improve patient comfort during procedures.
Ergonomic handles designed for clinician efficiency.
Advanced sterilization compatibility for infection control.
Digital recording and archiving of colonoscopy procedures.
Compatibility with hospital EMR systems.
Software upgrades for improved diagnostic accuracy.
AI-assisted lesion recognition for early detection of colorectal cancer.
The future of colonoscope procurement in hospitals will be driven by innovation, global supply chain dynamics, and evolving regulatory frameworks. Hospitals must adapt their procurement strategies to keep pace with emerging technologies while maintaining fiscal responsibility. Colonoscope OEM ODM models will remain critical for achieving scalability and flexibility.
4K and 8K imaging in colonoscopy equipment for ultra-high-resolution diagnostics.
Disposable colonoscopes reducing infection risks and sterilization costs.
Integration of AI software into colonoscopy systems for real-time clinical decision support.
Sustainability-focused colonoscope factories developing eco-friendly devices.
Asia-Pacific colonoscope factories will dominate in volume production, offering cost-effective solutions.
European colonoscope manufacturers will continue leading in innovation and regulatory compliance.
North American colonoscope suppliers will emphasize integrated healthcare system compatibility.
Latin American hospitals will increasingly adopt ODM solutions for localized customization.
By 2025 and beyond, colonoscope OEM ODM procurement will remain a vital strategy for hospitals seeking to combine affordability, quality, and clinical effectiveness. Hospitals that build long-term partnerships with colonoscope factories, colonoscope suppliers, and colonoscope manufacturers will secure sustainable access to advanced colonoscopy machines and colonoscopy systems, ensuring patient care excellence while managing costs effectively.
The global market for colonoscope OEM ODM in 2025 reveals significant differences across regions, influenced by local manufacturing capabilities, healthcare budgets, regulatory environments, and technological adoption. Hospitals worldwide must carefully consider these variables when evaluating colonoscope suppliers, colonoscope factories, and colonoscope manufacturers. Price, availability, and quality assurance vary widely, making regional analysis essential for procurement teams. The colonoscopy equipment sector has grown rapidly in the last decade due to the increasing prevalence of colorectal diseases and the global focus on preventive screening programs.
In North America, particularly in the United States and Canada, the colonoscopy system is regarded as a premium medical device category. Hospitals typically purchase colonoscopy machines from colonoscope manufacturers that strictly adhere to FDA approvals and advanced quality testing. Colonoscope suppliers in this region emphasize product integration with hospital information systems and provide long-term after-sales service packages. As a result, the colonoscope price in North America is among the highest worldwide, ranging from USD 3,800 to 5,500 for advanced video colonoscopes. Colonoscope factories in North America are fewer in number compared to Asia-Pacific, but their strong emphasis on R&D leads to innovative features such as AI-assisted diagnostics and 8K imaging.
Key Characteristics: High regulatory compliance, advanced colonoscopy equipment, premium colonoscope price.
Typical Suppliers: Large colonoscope manufacturers focusing on innovation and long-term hospital contracts.
Challenges: High costs limit adoption in smaller hospitals and rural clinics.
European hospitals adopt colonoscope OEM ODM procurement with a focus on balancing innovation, compliance, and affordability. Colonoscope manufacturers in Germany, France, and the UK are known for producing high-quality colonoscopy equipment with strong emphasis on ergonomics and sustainability. Colonoscope suppliers in Europe often highlight compliance with CE regulations and sustainable practices, such as eco-friendly sterilization processes. Colonoscope factories located in Eastern Europe offer competitive pricing, making them attractive for hospitals seeking lower colonoscope prices without sacrificing quality. Colonoscopy systems in Europe often integrate multilingual software interfaces to accommodate diverse healthcare markets.
Key Characteristics: Strong emphasis on CE compliance, sustainability, and ergonomic design.
Average Colonoscope Price: USD 3,000–5,200 depending on OEM vs ODM model.
Opportunities: ODM customization services tailored to small and mid-sized hospitals.
Asia-Pacific has emerged as the world’s leading production center for colonoscope equipment. Colonoscope factories in China, Japan, and South Korea account for a large percentage of global output, supplying hospitals and distributors worldwide. Colonoscope suppliers in this region offer the most competitive colonoscope prices, with flexible colonoscopy machine models ranging from USD 2,500 to 4,500. Colonoscope manufacturers in Japan emphasize high-end imaging technologies, while Chinese colonoscope factories dominate in volume production and ODM customization. Hospitals in Asia-Pacific often benefit from direct access to colonoscopy systems, reducing logistics costs and ensuring fast replacement cycles.
Strengths: Cost efficiency, ODM flexibility, high production capacity.
Weaknesses: Variability in quality among smaller colonoscope factories.
Trends: Increasing demand for disposable colonoscopy equipment and digital integration.
Latin America is experiencing steady growth in colonoscope OEM ODM procurement as governments invest in healthcare modernization. Hospitals across Brazil, Mexico, and Argentina are adopting colonoscopy systems as part of national screening programs. Colonoscope suppliers in this region often import from Asia-Pacific colonoscope factories due to favorable colonoscope price points. However, regional colonoscope manufacturers are beginning to emerge, offering ODM services tailored to local hospital requirements. Price sensitivity remains high, and hospitals frequently negotiate with colonoscope suppliers to achieve cost reductions for bulk colonoscopy machine orders.
Key Characteristics: Price-sensitive market, reliance on imported colonoscopy equipment, growing ODM services.
Average Colonoscope Price: USD 2,800–4,700, typically lower than Europe and North America.
In the Middle East and Africa, demand for colonoscopy systems is increasing as hospitals expand diagnostic capabilities. Colonoscope factories are limited in this region, so hospitals rely heavily on international colonoscope suppliers from Europe and Asia-Pacific. Colonoscope prices are often higher due to import taxes, logistics costs, and limited local distribution networks. However, government initiatives in Saudi Arabia, UAE, and South Africa are driving hospital procurement of colonoscopy equipment through bulk OEM ODM contracts. Colonoscope manufacturers targeting this region often establish partnerships with local distributors to improve supply chain efficiency.
Challenges: High import costs, limited local colonoscope factories, reliance on international suppliers.
Opportunities: ODM customization for region-specific needs, such as language interface and power supply compatibility.
| Region | Strengths | Weaknesses | Average Colonoscope Price |
|---|---|---|---|
| North America | Innovation, compliance, service | High colonoscope price, limited affordability | $3,800–$5,500 |
| Europe | Balance of quality & cost, CE compliance | Higher costs in Western Europe | $3,000–$5,200 |
| Asia-Pacific | Low colonoscope price, high production capacity | Variable quality among small factories | $2,500–$4,500 |
| Latin America | Growing adoption, ODM customization | Heavy reliance on imports | $2,800–$4,700 |
| Middle East & Africa | Government initiatives, new procurement | Import costs, weak supply chain | $3,000–$5,200 |
This regional breakdown highlights the importance of customizing procurement strategies. Hospitals must carefully evaluate colonoscope factories and colonoscope suppliers not only by colonoscope price but also by compliance, service, and integration with hospital IT systems. As colonoscopy equipment continues to evolve, global procurement strategies in 2025 will depend on aligning local needs with international colonoscope manufacturers that can deliver consistent, reliable colonoscopy systems.
Real-world hospital procurement decisions provide valuable insights into how colonoscope OEM and ODM strategies are applied in practice. Hospitals of varying sizes and budgets face different challenges when sourcing colonoscopy equipment, colonoscopy machines, and colonoscopy systems. By analyzing procurement practices across large teaching hospitals, regional hospitals, and specialized clinics, we can better understand the benefits and limitations of working with colonoscope factories, colonoscope suppliers, and colonoscope manufacturers in 2025.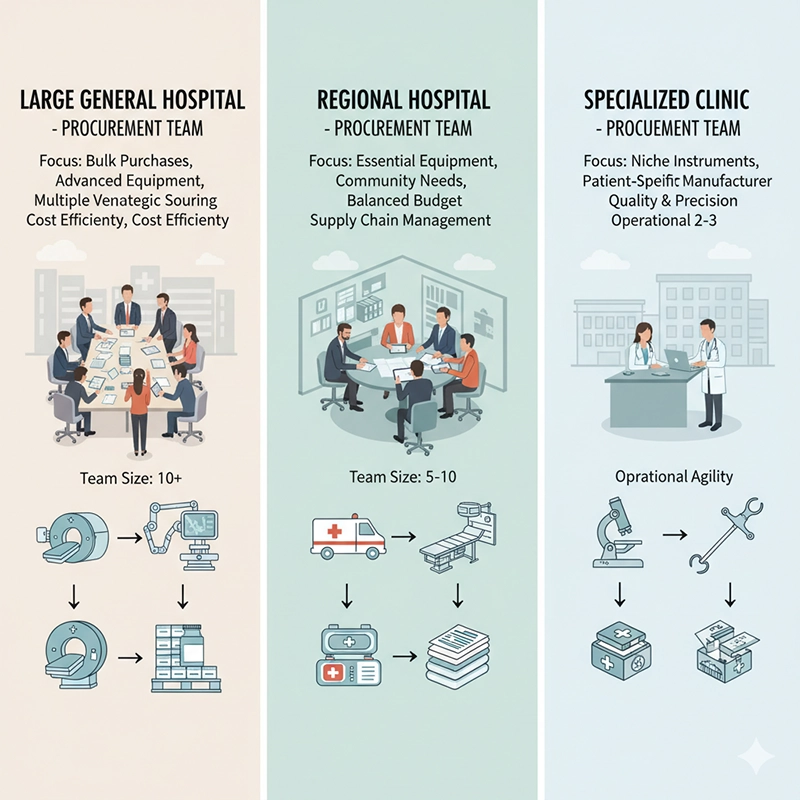
A large teaching hospital in North America recently implemented an OEM procurement strategy with a global colonoscope manufacturer. The hospital’s objective was to acquire colonoscopy systems with advanced imaging, AI-assisted diagnostics, and integration with electronic medical records (EMRs). This institution prioritized long-term partnerships with colonoscope suppliers that could guarantee consistent updates, training, and support.
Procurement Process: The hospital issued a detailed RFP (Request for Proposal) outlining technical specifications, colonoscope price ceilings, and regulatory compliance requirements.
Supplier Selection: Colonoscope factories with ISO13485 certification and FDA-approved colonoscopy machines were shortlisted.
OEM vs ODM Choice: The hospital chose an OEM model because it valued brand recognition, FDA clearance, and integration with existing IT infrastructure over customization.
Outcome: The hospital secured a five-year contract for colonoscopy equipment with a total cost of ownership model that included maintenance, staff training, and replacement warranties.
This case demonstrates how large hospitals with high patient volumes and advanced research programs prioritize innovation and regulatory compliance when working with colonoscope manufacturers. Although colonoscope price was a consideration, quality and service outweighed cost concerns.
A regional hospital in Eastern Europe faced budget constraints but needed to upgrade its outdated colonoscopy equipment. After evaluating multiple colonoscope suppliers, the hospital opted for an ODM partnership with a colonoscope factory in Asia-Pacific. This decision allowed the hospital to balance affordability with sufficient customization.
Procurement Process: The hospital evaluated colonoscope prices from European and Asian colonoscope manufacturers, comparing technical specifications and service packages.
Supplier Selection: An ODM colonoscope factory in China was selected because it offered cost-effective colonoscopy systems with CE certification and multilingual software interfaces.
OEM vs ODM Choice: The hospital selected an ODM model to incorporate specific features such as regional language support and adjustable sterilization settings compatible with local systems.
Outcome: Colonoscopy machines were delivered at 30% lower cost compared to European options, while service contracts were managed through a local distributor partnership.
This case illustrates how mid-sized hospitals often rely on colonoscope suppliers that provide flexibility in design and pricing. By choosing an ODM approach, the regional hospital achieved significant cost savings without sacrificing quality standards.
A gastroenterology clinic in Latin America adopted a unique procurement strategy by partnering with a colonoscope supplier offering disposable colonoscopes. This decision was driven by infection control priorities, limited sterilization facilities, and high patient turnover rates.
Procurement Process: The clinic reviewed colonoscope manufacturers that specialized in single-use colonoscopy machines, evaluating colonoscope price per unit and supply chain stability.
Supplier Selection: An ODM colonoscope factory was selected to produce customized disposable devices at scale.
OEM vs ODM Choice: The clinic opted for ODM services to include features such as pre-lubricated insertion tubes and lightweight handles to improve patient comfort.
Outcome: While the cost per unit colonoscope price was higher than reusable options, the clinic reduced sterilization costs and improved infection control, making the investment cost-effective in the long run.
This case highlights how smaller healthcare providers with specific needs may prioritize ODM colonoscope suppliers to address operational constraints. Disposable colonoscopy equipment, though initially more expensive, provided long-term value by improving efficiency and reducing hospital-acquired infection risks.
To better understand procurement strategies, hospitals often conduct cost-benefit analyses between OEM and ODM colonoscope models. OEM colonoscopy systems typically carry higher colonoscope prices but offer proven reliability, established service networks, and regulatory clearances. ODM colonoscope equipment provides flexibility, lower colonoscope prices, and opportunities for customization, though hospitals must carefully evaluate supplier quality.
| Procurement Model | Advantages | Disadvantages | Typical Use Case |
|---|---|---|---|
| OEM Colonoscope | Strong compliance, brand recognition, integrated support | Higher colonoscope price, less customization | Large teaching hospitals, research centers |
| ODM Colonoscope | Lower cost, design flexibility, localized features | Variable quality, requires careful supplier vetting | Regional hospitals, specialized clinics |
Large hospitals often prioritize innovation and regulatory compliance over colonoscope price.
Regional hospitals seek balance between colonoscope suppliers’ pricing and customization capabilities.
Specialized clinics may prefer ODM colonoscope manufacturers to address unique operational needs such as disposable colonoscopy systems.
OEM models ensure stability and compliance, while ODM models enhance flexibility and affordability.
These hospital procurement case studies demonstrate the diversity of strategies used worldwide. Colonoscope factories and colonoscope manufacturers must recognize that different healthcare facilities prioritize different aspects of procurement. As the market expands, hospitals will continue to diversify procurement approaches, leveraging both OEM and ODM colonoscope suppliers to optimize cost, quality, and clinical performance.
The supply chain for colonoscope OEM and ODM products in 2025 has become increasingly complex. Hospitals rely on colonoscope factories and colonoscope suppliers spread across multiple continents, making procurement highly sensitive to logistics disruptions, raw material shortages, and regulatory changes. To maintain access to colonoscopy equipment and colonoscopy systems, hospitals must evaluate not only colonoscope price but also supply chain resilience. Colonoscope manufacturers are under pressure to deliver innovation and consistency while navigating global challenges such as transportation delays, fluctuating raw material costs, and geopolitical uncertainties.
The majority of colonoscope factories are located in Asia-Pacific, with China, Japan, and South Korea accounting for a significant share of colonoscopy equipment production. Hospitals in North America, Europe, and Latin America frequently import colonoscopy machines from these manufacturing hubs. While this model reduces colonoscope price, it creates dependence on international logistics networks. Shipping delays caused by port congestion, customs clearance, or international conflicts can disrupt hospital procurement schedules.
Impact: Hospitals may face shortages of colonoscopy systems during peak demand.
Example: A European hospital network experienced a six-week delay in colonoscope deliveries due to shipping bottlenecks at major Asian ports.
Solution: Establish partnerships with colonoscope suppliers that maintain regional warehouses for faster distribution.
Colonoscope manufacturers rely on specialized raw materials, including high-quality optical glass, medical-grade plastics, and micro-electronic components. Global shortages of semiconductors and advanced sensors have affected colonoscopy equipment production. These shortages can increase colonoscope price and reduce the availability of specific colonoscopy machine models.
Impact: Colonoscope factories may extend lead times from 60 days to over 120 days.
Example: A colonoscope supplier in North America reported a 20% increase in procurement costs due to rising prices of imported imaging sensors.
Solution: Hospitals can negotiate long-term contracts with colonoscope manufacturers that secure raw material allocations in advance.
Colonoscope suppliers must comply with diverse regulatory frameworks, including FDA in the United States, CE in Europe, and CFDA in China. Hospitals working with colonoscope factories across multiple regions face the challenge of ensuring that colonoscopy systems meet all applicable standards. This creates complexity in procurement and increases the time required to validate colonoscopy equipment.
Impact: Delays in certification can prevent hospitals from deploying new colonoscopy machines on schedule.
Example: A Latin American hospital received colonoscope shipments from Asia-Pacific, but customs authorities delayed clearance due to missing CE documentation.
Solution: Hospitals should prioritize colonoscope manufacturers with global regulatory approvals and transparent compliance records.
Colonoscopy equipment requires regular maintenance, calibration, and sterilization. Hospitals depend on colonoscope suppliers to provide timely service and spare parts. However, when colonoscope factories are located overseas, after-sales service can be delayed, causing operational inefficiencies. This is particularly challenging for hospitals in remote regions without access to local colonoscope manufacturers or trained technicians.
Impact: Delays in maintenance may force hospitals to postpone colonoscopy procedures, affecting patient care.
Example: A hospital in Africa reported downtime of over two months for its colonoscopy system due to slow delivery of replacement parts.
Solution: Hospitals should negotiate comprehensive service contracts with colonoscope suppliers, including guaranteed spare parts availability and on-site training for biomedical engineers.
Events such as pandemics, geopolitical conflicts, and natural disasters can severely impact the colonoscope supply chain. Colonoscope manufacturers in affected regions may halt production, and logistics delays can escalate colonoscope prices worldwide. Hospitals with limited procurement flexibility are most vulnerable during such crises.
Impact: Colonoscopy machine shortages force hospitals to delay preventive screening programs.
Example: During the COVID-19 pandemic, colonoscope factories in Asia temporarily shut down, leading to backlogs in orders from North America and Europe.
Solution: Hospitals should diversify procurement sources by engaging multiple colonoscope suppliers across different regions.
Despite these challenges, hospitals can strengthen their procurement strategies by adopting proactive measures. Working with colonoscope factories and colonoscope manufacturers that emphasize transparency, flexibility, and digital integration can significantly improve supply chain stability. Procurement teams must consider not only colonoscope price but also long-term risk management.
Hospitals should avoid dependence on a single colonoscope supplier. By partnering with multiple colonoscope manufacturers in different regions, hospitals reduce the risk of supply chain disruptions. This approach also increases bargaining power, leading to more competitive colonoscope prices.
Leading colonoscope factories now maintain regional stock points to minimize shipping delays. Hospitals working with colonoscope suppliers that operate local warehouses benefit from shorter delivery times, reduced customs risks, and faster replacement of colonoscopy equipment.
Colonoscope manufacturers increasingly deploy digital platforms for tracking orders, monitoring logistics, and forecasting inventory needs. Hospitals that adopt digital procurement systems gain visibility into colonoscope factory production schedules and shipping timelines, ensuring smoother procurement of colonoscopy systems.
Hospitals can secure stability by negotiating long-term OEM or ODM agreements with colonoscope factories. These contracts guarantee access to colonoscopy machines at fixed colonoscope prices, protect against market fluctuations, and ensure priority delivery during global crises.
Hospitals must invest in local capacity building by training biomedical engineers to handle colonoscopy equipment repairs. Colonoscope manufacturers can support this by providing technical manuals, online support, and regional training centers. This reduces downtime and dependency on international technicians.
North America: A hospital group signed multi-year OEM contracts with two colonoscope manufacturers to secure stable colonoscopy system supply during semiconductor shortages.
Europe: Regional colonoscope suppliers established distribution hubs in Germany to reduce customs delays for EU hospitals.
Asia-Pacific: Colonoscope factories in China introduced blockchain-based tracking for colonoscopy machine shipments, improving transparency.
Latin America: Hospitals partnered with local colonoscope suppliers that stock spare parts, reducing equipment downtime from months to weeks.
Africa: Colonoscope manufacturers set up regional training programs for hospital technicians, minimizing delays in maintenance and calibration.
In conclusion, colonoscope OEM ODM procurement in 2025 requires hospitals to look beyond colonoscope price. By addressing supply chain challenges through multi-supplier strategies, digital integration, and regional partnerships, hospitals can ensure reliable access to colonoscopy equipment. Colonoscope factories and colonoscope manufacturers that prioritize resilience and transparency will emerge as preferred partners for global hospital procurement teams.
As hospitals adapt to new realities in 2025, colonoscope OEM ODM procurement is evolving to incorporate cutting-edge technologies, sustainable manufacturing practices, and data-driven supply chain management. Colonoscope factories and colonoscope manufacturers must innovate not only in colonoscopy equipment design but also in how they support hospitals with scalable, customizable colonoscopy systems. Future procurement strategies will emphasize advanced imaging, AI integration, sustainability, and value-based pricing models, ensuring hospitals can balance colonoscope price with clinical excellence and patient safety.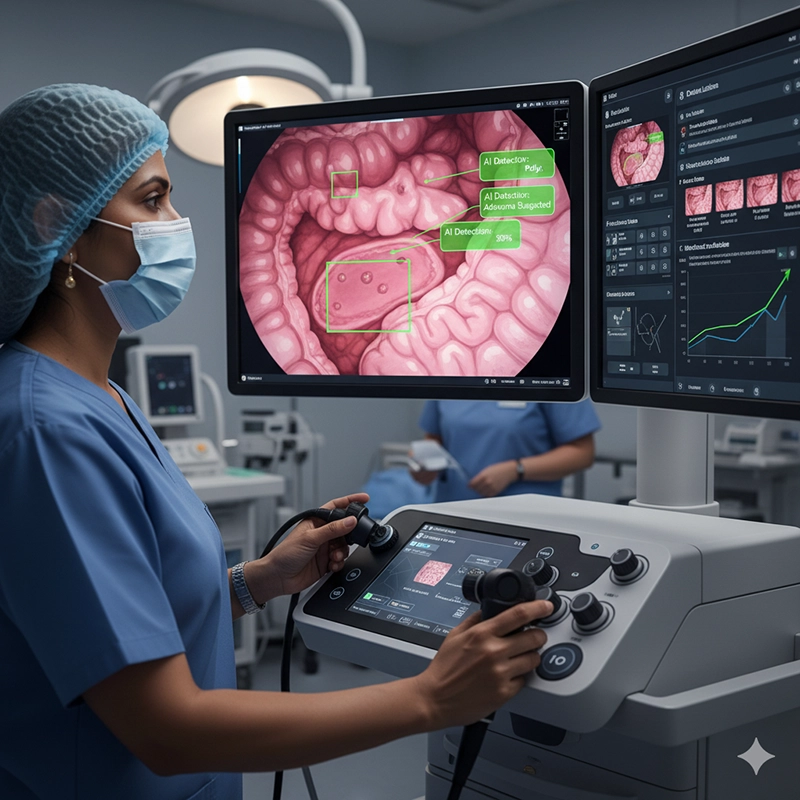
Artificial intelligence is transforming the colonoscopy machine market by enabling real-time lesion detection, automated polyp classification, and predictive analytics for patient outcomes. Hospitals increasingly demand colonoscopy systems equipped with AI modules that assist physicians during procedures. Colonoscope manufacturers are responding by embedding AI algorithms into OEM designs, while ODM colonoscope suppliers offer customization options tailored to local clinical guidelines.
Clinical Benefit: AI improves diagnostic accuracy, reducing missed polyps by up to 25% according to studies published by medical associations.
Procurement Impact: Hospitals view AI-enabled colonoscopy equipment as an investment that reduces long-term costs by improving early detection and preventing complications.
Supplier Strategy: Colonoscope factories offering AI modules gain a competitive edge in hospital procurement negotiations.
Infection control remains a top priority for hospitals. Colonoscope suppliers are introducing single-use colonoscopy machines that eliminate the risk of cross-contamination. Colonoscope factories producing disposable devices are particularly attractive to specialized clinics with limited sterilization infrastructure. While the colonoscope price per unit is higher, hospitals achieve savings by avoiding sterilization costs and reducing hospital-acquired infections.
Advantage: Single-use colonoscopy systems reduce downtime and maintenance needs.
Limitation: Higher recurring costs for hospitals with high patient volumes.
Market Trend: ODM colonoscope manufacturers are expanding disposable device portfolios for clinics in Latin America and Africa.
Sustainability is becoming a key consideration in colonoscope OEM ODM procurement. Colonoscope manufacturers are investing in eco-friendly colonoscope factories that minimize energy consumption and incorporate recyclable materials into colonoscopy equipment. Hospitals increasingly consider environmental impact when selecting colonoscope suppliers, aligning procurement policies with global sustainability goals.
Example: A European colonoscope factory adopted biodegradable plastics in colonoscopy machine handles, reducing medical waste by 15%.
Procurement Impact: Hospitals seeking “green procurement” strategies view eco-friendly colonoscope manufacturers as long-term partners.
Future Outlook: Sustainability certifications may soon accompany ISO and CE requirements in procurement contracts.
Hospitals demand colonoscopy systems that integrate seamlessly with electronic medical records (EMR) and hospital information systems (HIS). Colonoscope manufacturers are developing software-enabled colonoscopy equipment that allows physicians to record, store, and analyze patient data in real time. This trend improves efficiency and supports data-driven decision-making.
Clinical Value: Integrated colonoscopy systems streamline workflows and enhance reporting accuracy.
Procurement Consideration: Hospitals must evaluate whether colonoscope suppliers provide long-term software updates and cybersecurity measures.
Supplier Strategy: Colonoscope factories offering IT integration gain preference in high-volume procurement contracts.
Traditional procurement models are evolving as hospitals negotiate value-based pricing with colonoscope suppliers. Instead of paying solely for equipment, hospitals now seek contracts that include maintenance, training, and spare parts within the colonoscope price. Colonoscope manufacturers offering subscription-based models for colonoscopy equipment are gaining traction, particularly among regional hospitals with budget constraints.
| Pricing Model | Description | Hospital Benefit |
|---|---|---|
| Traditional Purchase | Upfront payment for colonoscopy machines | Full ownership, but high initial cost |
| Leasing | Monthly payments for colonoscopy equipment use | Lower initial cost, flexibility to upgrade |
| Subscription | Comprehensive contract covering equipment, service, and upgrades | Predictable expenses, reduced operational risk |
By 2025 and beyond, colonoscope OEM ODM procurement will become more dynamic, blending technological innovation, sustainable practices, and flexible business models. Hospitals must adapt by building strategic partnerships with colonoscope factories and colonoscope suppliers that not only deliver cost-effective colonoscopy systems but also anticipate future healthcare needs. Colonoscope manufacturers capable of integrating AI, developing eco-friendly devices, and offering advanced service models will lead the market.
Develop multi-supplier procurement networks to reduce reliance on single colonoscope factories.
Prioritize colonoscope manufacturers offering AI-enabled and IT-integrated colonoscopy equipment.
Negotiate value-based contracts that include service, training, and spare parts.
Adopt sustainable procurement policies by choosing eco-friendly colonoscope suppliers.
Monitor colonoscope price trends globally to balance cost efficiency and quality.
Hospital procurement strategies for colonoscope in 2025 highlight a clear shift toward smarter, more resilient, and patient-centered models. Colonoscope factories, colonoscope suppliers, and colonoscope manufacturers must align their production and service models with the evolving demands of hospitals worldwide. Whether through AI-enhanced colonoscopy systems, disposable colonoscopy equipment, or eco-friendly manufacturing, innovation will drive the next wave of procurement decisions. Hospitals that embrace diversified supplier networks, value-based contracts, and sustainable practices will be best positioned to deliver high-quality patient care while controlling colonoscope prices and ensuring long-term access to critical medical technology. The integration of technology, cost efficiency, and global collaboration marks the future of colonoscopy equipment procurement, creating a healthcare landscape that is both advanced and accessible.
Yes, we offer OEM colonoscope equipment that can be tailored to hospital specifications, including imaging resolution, ergonomics, and sterilization compatibility.
Our factory provides ODM colonoscopy systems, allowing hospitals to integrate local language software, AI diagnostic modules, and specific sterilization settings.
Depending on the model and customization, colonoscope prices for OEM orders range from USD 2,500–5,000 per unit, with discounts for large procurement volumes.
Each colonoscope passes multi-stage inspections, including optical testing, sterilization validation, and clinical trial evaluations, before shipment.
Standard delivery is 60–90 days, depending on customization requirements and order quantity.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS