Table of Contents
When hospitals, clinics, and distributors evaluate how to choose an endoscope factory, the decision centers on product quality, regulatory compliance, manufacturing capability, after-sales service, and long-term supply reliability. Procurement teams must weigh certifications, technological expertise, customization options, and pricing structures to identify a partner that aligns with both clinical needs and budget goals. Choosing the right factory ensures consistent device performance, supports minimally invasive procedures, and reduces risks of supply interruptions—making it one of the most strategic choices in modern healthcare equipment sourcing.
Endoscopy plays a crucial role in modern medicine, from routine diagnostic checks to complex surgical interventions. The factory where endoscopes are designed and manufactured directly determines product safety, durability, and imaging clarity. Unlike general medical supplies, endoscopes are precision instruments with intricate optics, miniaturized components, and advanced imaging processors.
Procurement managers and clinicians therefore face a decision that affects patient outcomes, operational efficiency, and institutional reputation. A poor choice in factory can lead to delayed deliveries, high maintenance costs, or even patient safety issues, while a trusted endoscope factory becomes a long-term partner in advancing healthcare delivery.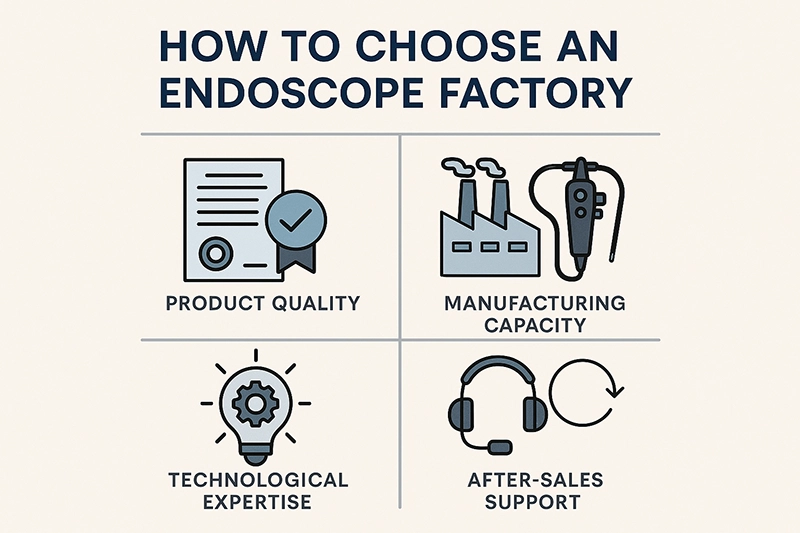
The first criterion is the overall quality of the endoscope. Factories should demonstrate rigorous quality assurance processes, consistent raw material sourcing, and in-house testing protocols. High-definition imaging, ergonomic handling, and reliable sterilization compatibility distinguish reputable products. Buyers should request product testing data, references from current hospital clients, and evidence of performance in demanding clinical settings.
Medical devices must comply with stringent international standards. The most reputable endoscope factories will hold certifications such as:
ISO 13485: Quality management system for medical devices.
CE Marking: Compliance with European regulatory requirements.
FDA Registration: Approval for the U.S. market.
RoHS Compliance: Restriction of hazardous substances in electronic components.
Certification demonstrates not just legal compliance but also the factory’s commitment to global best practices.
A factory’s ability to handle large-volume orders without compromising quality is critical. Procurement teams should examine production lines, automation systems, and supply chain resilience. During peak demand—such as global health crises—factories with scalable capacity ensure that hospitals do not face critical shortages of essential devices.
Technology in endoscopy evolves rapidly, with innovations like 4K imaging, narrow band imaging (NBI), AI-assisted lesion detection, and ultra-thin insertion tubes. An advanced factory invests heavily in research and development, enabling continuous upgrades and adaptation to emerging clinical needs. This innovative edge is essential for institutions looking to stay competitive and improve diagnostic accuracy.
Many hospitals and distributors seek OEM (Original Equipment Manufacturer) or ODM (Original Design Manufacturer) solutions. A flexible factory can customize branding, specifications, or entire system integrations according to client requirements. This flexibility helps distributors expand market presence and hospitals secure equipment that precisely matches departmental workflows.
Price remains a key factor in choosing an endoscope factory. However, the lowest quote rarely guarantees long-term value. Buyers must compare total cost of ownership (TCO), which includes:
Initial purchase price
Maintenance and repair costs
Training and installation fees
Spare parts availability
Product lifespan
A factory that balances competitive pricing with durability often provides the best return on investment for procurement teams.
To make an informed decision, procurement managers should prepare structured evaluation questions, such as:
What certifications does your facility currently hold?
Can you provide references from international hospitals or distributors?
How do you test optical clarity, flexibility, and durability before shipment?
What is your standard lead time for bulk orders?
Do you provide training for medical staff in endoscope use and care?
What after-sales services and warranty coverage do you offer?
How do you ensure continuity of supply during global supply chain disruptions?
The answers to these questions reveal not only technical competence but also the factory’s willingness to act as a long-term partner.
Endoscopes require regular maintenance, reprocessing, and occasional repairs. A reliable factory provides:
On-site training for nurses and technicians.
Global service centers or partnerships with regional distributors.
Quick turnaround times for repairs.
Availability of spare parts for both current and legacy models.
Without this support, hospitals face downtime that can delay urgent diagnostic or surgical procedures.
Choosing between domestic factories and international suppliers often depends on budget, shipping times, and regulatory requirements.
Domestic factories: Faster delivery, easier communication, and simpler compliance with national regulations.
International factories (e.g., Asia, Europe): Often offer lower costs and broader technological options but may involve longer lead times and higher shipping fees.
A balanced strategy is to combine domestic purchases for urgent needs with international sourcing for cost efficiency and advanced technology access.
Many healthcare institutions report that factory partnerships directly affect clinical workflow. For example:
Hospitals that sourced from factories with strong R&D capabilities adopted 4K endoscopy earlier, improving cancer detection rates.
Distributors working with flexible OEM factories expanded product portfolios under private labels, gaining competitive market share.
Facilities that partnered with poorly managed factories suffered from inconsistent deliveries, leading to operational bottlenecks.
These cases highlight the tangible impact of factory selection on healthcare outcomes and business performance.
Integration of AI for image recognition
Sustainable production methods reducing environmental impact
Smart endoscopes with cloud connectivity
Miniaturization of scopes for pediatric and delicate procedures
Factories leading in these innovations are more likely to remain reliable partners for the next decade.
Digital manufacturing platforms—such as Industry 4.0 automation, digital twins, and AI-driven quality inspection—enhance precision and efficiency. Buyers should prioritize factories adopting these digital tools, as they minimize defects, improve traceability, and shorten production cycles.
Choosing an endoscope factory is not a one-time purchase decision but the beginning of a multi-year collaboration. Strong partnerships are built on:
Transparent communication
Reliable supply schedules
Shared commitment to innovation
Continuous feedback between clinicians and engineers
Factories that embrace collaborative relationships create a foundation for sustainable healthcare solutions.
1. Verify ISO 13485, CE, FDA, and RoHS certifications.
2. Review product quality reports and clinical references.
3. Assess R&D and innovation capabilities.
4. Evaluate OEM/ODM customization options.
5. Compare total cost of ownership, not just unit price.
6. Confirm after-sales support and training.
7. Inspect manufacturing capacity and scalability.
8. Consider geographic factors and shipping timelines.
9. Review digitalization and automation levels.
10. Build for long-term partnership potential.
Selecting the right endoscope factory involves balancing quality, compliance, cost efficiency, and innovation. It is a strategic procurement decision with direct consequences for patient care and institutional reputation. Hospitals, clinics, and distributors should approach the process with structured evaluations, in-depth factory audits, and a focus on long-term reliability. By applying these principles, healthcare organizations can secure endoscope systems that consistently deliver safe, effective, and modern clinical performance.
Copyright © 2025.Geekvalue All rights reserved.Technical Support:TiaoQingCMS